Kinetic Theory
Get insights from 102 questions on Kinetic Theory, answered by students, alumni, and experts. You may also ask and answer any question you like about Kinetic Theory
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
According to KTG, the gas exerts pressure because its molecule :
Suffer change in momentum when impinge on the walls of container.
New answer posted
5 months agoContributor-Level 10
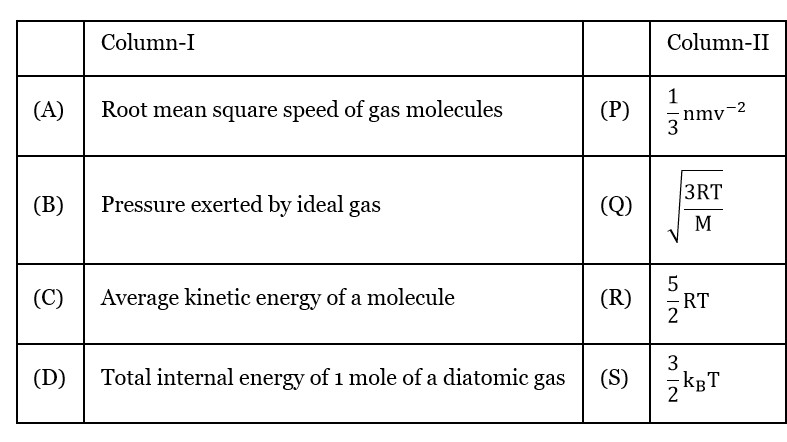
Root mean square speed of gas molecules
Pressure exerted by ideal Gas
Average kinetic energy of a molecular
Total internal energy of 1 mole of a diatomic gas
(For 1 mole diatomic gas)
New answer posted
5 months agoContributor-Level 10
v_rms = √ (3RT/M) and v_av = √ (8RT/πM)
⇒ v_rms / v_av = √ [ (3RT/M) / (8RT/πM)] = √ (3π/8)
New answer posted
6 months agoContributor-Level 9
According to principle of equi-partition of Energy, the average energy per molecules associated with each degree of freedom is (1/2)kT.
New answer posted
6 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers