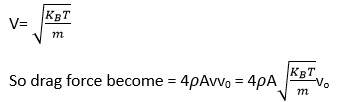Kinetic Theory
Get insights from 102 questions on Kinetic Theory, answered by students, alumni, and experts. You may also ask and answer any question you like about Kinetic Theory
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Consider the diagram
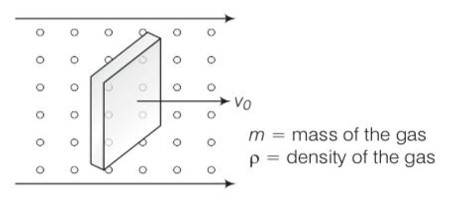
let n =number of molecules per unit volume
Vrms= rms speed of gas molecule
When block is moving with speed vo, relative speed of molecules w.r.t front face =v+vo
Coming head on, momentum transferred to block per collision =2m (v+vo)
Number of collisions in time = (v+vo)n A where A is the area of cross section.
So momentum transferred in time =m (v+vo)2nA this is from front surface
Similarly momentum transferred in time = m (v-vo)2nA ) this is from back surface
Drag force = mnA (v+vo)2- (v-vo)2)
= mnA (4wo)=4mnAvvo
= 4 vvo
So =mn/V=M/
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
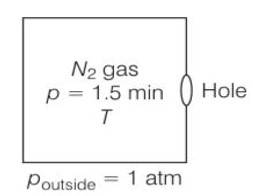
Given volume V = 1m3
area = 0.01mm2
= 8.01 m2= m2
Temperature both inside and outside are equal
So initial pressure inside the box = 1.50atm
Final pressure inside the box= 0.1atm
Assuming Vx= speed of nitrogen molecule in x direction
ni = number of molecules per unit volume in a time interval of
Let area of the wall, number of particles colliding in time
= i (vx )A , here we use ½ because particle moves both in positive and negative direction.
Vx2+ Vy2+ Vz2= Vrms2
Vx2= Vrms2/3 if all three velocities are equal.
½ mvrms2= 3/2KBT/m


New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Time require to avoid the collision T= l/v where l = mean free path =1/
Where n = N/V
n=number of aeroplanes/volume
= -3
T=
T= =
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
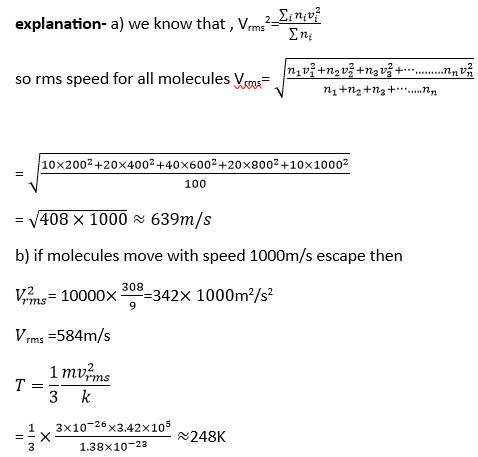
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(a) The moon has small gravitational force and hence, the escape velocity is small .
As the moon in the proximity of the earth as seen from the sun, the moonhas the same amount of heat per unit area as that of the earth.
The air molecules have large range of speeds . even though the rms speed of the air molcules is smaller than the escape velocity on the moon, a significant number of molecules have speed grater than the escape velocity.
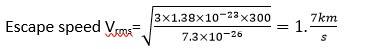
(b) As the molecules move higher their potential energy increases and hence kinetic energy decreases and hence temperature reduces. At g
New answer posted
9 months agoContributor-Level 10
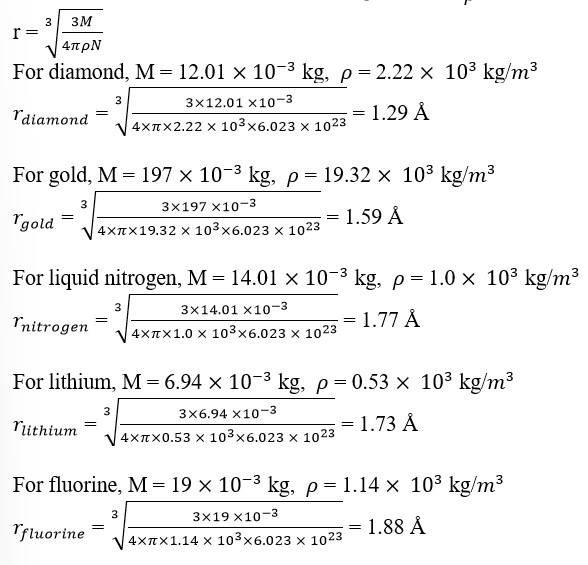
13.14 Let the atomic mass of a substance be = M and the density of the substance be =
Avogadro's number, N = 6.023
Volume of N number of molecules = ……. (i)
Volume of one mole of a substance = …… (ii)
Equating (i) and (ii), we get
New answer posted
9 months agoContributor-Level 10
13.13 According to law of atmospheres, we have
n2 = n1 exp [ -mg (h2 – h1)/ kBT] ….(i)
where is the number of density at height and is the number of density at height
mg is the weight of the particle suspended in the gas column
Density of the medium =
Density of the suspended particle =
Mass of one suspended particle = m'
Mass of medium displaced = m
Volume of the suspended particle = V
According to Archimedes's principle for a particle suspended in a liquid column, the effective weight of the suspended particle is given as:
Weight of the medium displaced – weight of the suspended particle = mg – m'g
= mg- V = mg –
New answer posted
9 months agoContributor-Level 10
13.12 Rate of diffusion of hydrogen, = 28.7 cm3 s–1
Rate of diffusion of another gas, = 7.2 cm3 s–1
According to Graham's law of diffusion, we have:
= , where = molecular mass of hydrogen = 2.02 g and is the molecular mass of the unknown gas
= 2.02= 32.09 = Molecular mass of Oxygen
Hence, the unknown gas is Oxygen.
New answer posted
9 months agoContributor-Level 10
13.11 Length of the narrow bore, L = 1 m = 100 cm
Length of the mercury thread, l = 76 cm
Length of the air column between mercury and the closed end, = 15 cm
Since the bore is held vertically in air with the open end at the bottom, the mercury length that occupies the air space is 100 – (76 + 15) = 9 cm
Hence, total length of the air column = 15 + 9 = 24 cm
Let h cm of mercury flow out as a result of atmospheric pressure.
Length of the air column in the bore = 24 + h cm
Length of the mercury column = 76 – h cm
Initial pressure, = 76 cm of mercury
Initial volume, = 15
Final pressure, = 76 – (76 – h) = h cm
New answer posted
9 months agoContributor-Level 10
13.10 Pressure inside the cylinder containing nitrogen, P = 2.0 atm = 2 Pa
Temperature inside the cylinder, T = 17
Radius of nitrogen molecule, r = 1.0 Å = 1 m
Diameter of nitrogen molecule, d = 2 m
Molecular mass of nitrogen molecule, M = 28 u = 28 g (assume) = 28 kg
The root means square speed of nitrogen is given by the relation

R is the universal gas constant = 8.314 J/mole/K
Hence
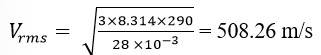
The mean free path is given by
where k = Boltzmann constant = 1.38 kg-
= 1.11 m
Collision frequency = = = 4.57 /s
Collision time, T = =S= 3.93 s
T
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers