Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
3.52. Ionization enthalpy represents the energy required to remove an electron from an isolated gaseous atom in its ground state. Nitrogen has a greater ionization enthalpy than that of oxygen due to its exactly half-filled p-orbitals.
New answer posted
6 months agoContributor-Level 10
(d) Chlorofluorocarbons (CFCs) are man-made industrial chemicals used in air conditioning etc.
New answer posted
6 months agoContributor-Level 10
3.51. Modern Periodic Law states that physical and chemical properties of the elements are a periodic function of their atomic numbers.
New answer posted
6 months agoContributor-Level 10
(b) The compound contaminates the ground water and is also a suspected carcinogen.
New answer posted
6 months agoContributor-Level 10
(b) CO is highly poisonous to living beings because of its ability to block the delivery of oxygen to the organs and tissues.
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
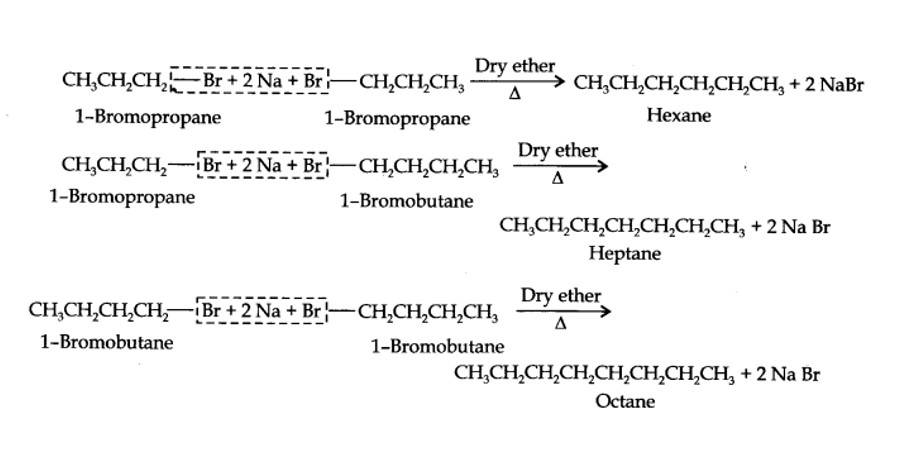
Alkyl halides on treatment with sodium metal in dry ethereal (free from moisture) solution give higher alkanes. This reaction is known as Wurtz reaction and is used for the preparation of higher alkanes containing even number of carbon atoms.
For preparation of alkanes containing odd number of carbon atoms, a mixture of two alkyl halides has to be used. Since two alkyl halides can react in three different ways, therefore, a mixture of three alkanes instead of the desired alkane would be formed. For example, Wurtz reaction between 1-bromopropane and 1-bromobutane gives a mixture of three alkanes i.e., hexane, heptane and octane as shown
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers