Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
3.46. (b) Electronegativity refers to the tendency of an atom to share electrons with another atom.
New answer posted
6 months agoContributor-Level 10
Anhydrous Ferric Chloride (FeCl3) is another Lewis acid which can be used during ethylation of benzene.
New answer posted
6 months agoContributor-Level 10
CH3 group is electron-donating while -NO2 group is electron-withdrawing. Therefore, maximum electron density will be in toluene, followed by benzene and least in m-dinitrobenzene. Therefore, the ease of nitration decreases in the order: toluene > benzene > m-dinitrobenzene.
New answer posted
6 months agoContributor-Level 10
(a) The typical reactions of benzene are electrophilic substitution reactions. Higher the electron-density in the benzene ring, more reactive is the compound towards thesereactions. Since NO2 is a more powerful electron-withdrawing group than Cl, therefore, more the number of nitro groups, less reactive is the compound. Thus, the overall reactivity decreases in the order:Chlorobenzene > p-nitrochlorobenzene > 2, 4-dinitrochlorobenzene
(b) Here, CH3 group is electron donating but NO2 group is electron-withdrawing. Therefore, the maximum electron-density will be in toluene, followed by p-nitrotoluene followed by p-dinitrobenzene. Thus,
New answer posted
6 months agoContributor-Level 10
3.45. (b) It has only ones-electron and hence can be placed in group 1 (alkali metals). It can also gain an electron to achieve a noble gas arrangement and hence it can behave similarly to a group 17 (halogen family) element. Because it is a special case, we shall place hydrogen separately at the top of the Periodic Table
New answer posted
6 months agoContributor-Level 10
The basic skeleton structure of 2-methylbutane is
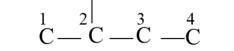
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
Benzene is a rich source of electrons because of the presence of an electron cloud containing 6 n-electrons above and below the plane of the ring. Consequently, it attracts the electrophiles (electron-deficient) reagents towards it and repels nucleophiles (electron- rich) reagents. As a result, benzene undergoes electrophilic substitution reactions easily and nucleophilic substitutions with difficulty.
New answer posted
6 months agoContributor-Level 10
3.44. (d) Assertion is a wrong statement. Non-metallic elements have a strong tendency to gain electrons. Therefore, electronegativity is directly related to those non-metallic properties of elements. It can be further extended to say that the electronegativity is inversely related to the metallic properties of elements.
Thus, the increase in electronegativities across a period is accompanied by an increase in non-metallic properties (or a decrease in metallic properties) of elements
New answer posted
6 months agoContributor-Level 10
The hybridization state of carbon in these three compounds is:
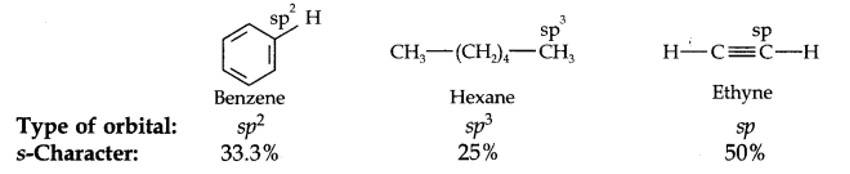
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
