Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Diamond has a crystalline lattice where each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in space and produces a rigid three- dimensional network of carbon atoms. It is very difficult to break extended covalent bonding and, therefore, diamond is a the hardest substance on the earth.
Graphite has layered structure in which the layers are held by van there Waals forces and distance between two layers is 340 pm. Each layer is composed of planar hexagonal rings of carbon atoms. C—C bond length
New answer posted
6 months agoContributor-Level 10
1.4 In order to answer the question, we need to know the balanced equation for the combustion of carbon in dioxygen/air, which can be written as:
C (s) + O2 (g) à CO2 (g)
(i) We can see from the above equation,
1 mole of carbon reacts with 1 mole of oxygen to produce 1 mol of carbon dioxide.
In air, combustion is complete.
Therefore, CO2 produced from combustion of 1 mole of carbon= Molar mass of CO2= 44 g
(ii) As only 16 g of dioxygen is available, it can combine only with 0.5 mole of carbon, i.e., dioxygen is the limiting reactant.
Hence, CO2 produced = 22 g
Here, dioxygen acts as the limiting reagent.
(iii) Here again, dioxygen is
New answer posted
6 months agoContributor-Level 10
1.3 We are given that the percentage of iron by mass is 69.9% and the percentage of oxygen by mass is 30.1%.
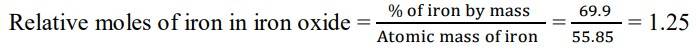
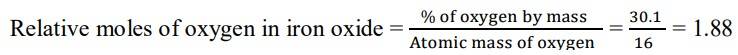
Since we have relative moles of both elements, we can calculate the simpler molar ratio of iron to oxygen
= 1.25: 1.88
Divide by the smaller value to both
= 1.25/1.25: 1.88/1.25
= 1: 1.5
= 2: 3
So, now we can write the empirical formula of iron oxide as Fe2O3.
New question posted
6 months agoNew answer posted
6 months ago11.9. What are electron deficient compounds? Are BCl3 and SiCl4 electron deficient species? Explain.
Contributor-Level 10
Electron deficient compounds are those in which the central atom in their molecule has the tendency to accept one or more electron pairs. They are also known as Lewis acid. BCl3 and SiCl4 both are electron deficient species.
Since, in BCl3, B atom has only six electrons. Therefore, it is an electron deficient compound.
In SiCl4 the central atom Si has 8 electrons but it can expand its covalency beyond 4 due to the presence of d-orbitals.
New answer posted
6 months agoContributor-Level 10
Aluminium reacts with acid as well as base. This shows amphoteric nature of aluminium.
2Al (s) + 6HCl (dil.) →2AlCl3 (aq) + 3H2 (g)
2Al (s) + 2NaOH (aq) + 6H2O (l) →2Na+ [Al (OH)4]– (aq) + 3H2 (g)
New answer posted
6 months agoContributor-Level 10
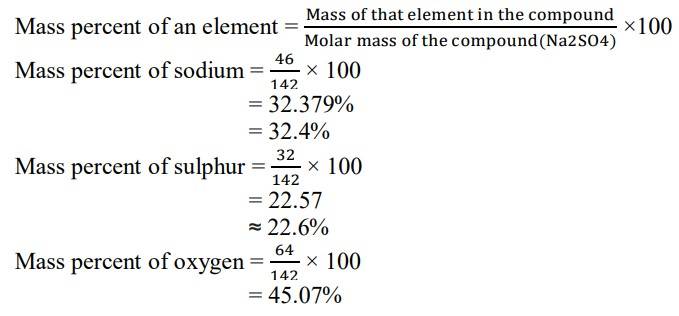
1.2 Molar mass of Na2SO4= (2 x Atomic mass of Sodium) + Atomic mass of Sulphur + (4 x Atomic mass of Oxygen)
= (2x 23) + 32 + (4x 16)
= 46 + 32 + 64
= 142 g/mol
New answer posted
6 months agoContributor-Level 10
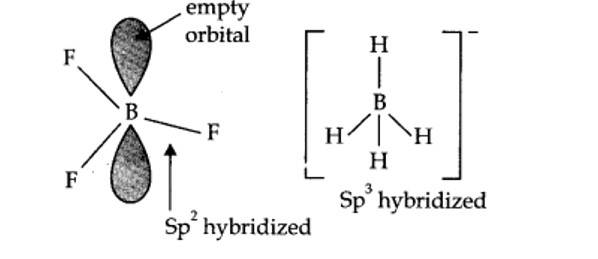
In BF3, boron is sp2 hybridized.
? shape of BF3 = planar.
In [BH4]–, boron is sp3 hybridized, thus the shape is tetrahedral.
New answer posted
6 months agoContributor-Level 10
On heating boric acid above 370 K, it forms metaboric acid, HBO2 which on further heating yields boric oxide B2O3.
H3B2O3 → HBO2 → B2O3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
