Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Hydrogen peroxide cannot be concentrated by heating as it can cause bumps on heating. It can be extracted with water and concentrated to ~30% (by mass) by distillation under reduced pressure. It can be further concentrated to ~85% by careful distillation under low pressure. The remaining water can be frozen out to obtain pure H2O2.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Water has a significantly higher boiling point than hydrogen sulphide because of the presence of strong hydrogen bonds between water molecules, which are absent in hydrogen sulphide due to the lower electronegativity of sulfur compared to oxygen; this means more energy is required to separate water molecules, resulting in a higher boiling point.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
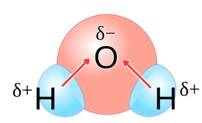
Water molecules are polar because in the gas phase water is a bent molecule with a bond angle of 104.5°, O-H bond length of 95.7 pm. Oxygen is more elsectronegative (EN = 3.5 ) than hydrogen (EN= 2.1 ) hence, O-H bond is polar . In the water molecule, two polar O-H nonds area present which are held together at an angle of 104.5 . Due to the resultant of these two dipoles, water molecule is polar and has an dipole moment of 1.84 Debye.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
H2O2 decomposes slowly on exposure to light.
2H2O2→ 2H2O+2O2
In daily life it is used as a hair bleach and as a mild disinfectant. As an antiseptic it is sold in the market as perhydrol.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(i) Oxidising action in acidic medium:
2Fe2+ (aq)+2H+ (aq)+H2O2 (aq) → 2Fe3+ (aq)+2H2O (l)
PbS (s)+4H2O2 (aq) → PbSO4 (s)+4H2O (l)
(ii) Reducing action in acidic medium:
2MnO4-+6H++5H2O2 → 2Mn2++8H2O+5O2
HOCl+H2O2 → H3O++Cl-+H2O
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
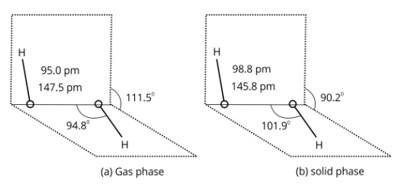
H2O2 structure in gas phase, dihedral angle is 111.50. H2O2 structure in solid phase at 110 k, dihedral angle is 90.20.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Heavy water can be used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Hydrogen economy (Hydrogen as fuel):
(i) The electricity cannot be stored in automobiles. It is not possible to store and transport nuclear energy. Hydrogen is an alternative source of energy and hence called the 'hydrogen economy". Hydrogen has some advantages as fuel.
(ii) Available in abundance in combined form as water.
(iii) On combustion produces H2O. Hence, pollution free.
(iv) H2O2 fuel cells give more power.
(v) Excellent reducing agent. Therefore, it can be used as a substitute of carbon in reduction for processes in industry.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar\
In sodium the last shell electron is in 3s1 after losing that electron, it can acquire the configuration of Ne. Whereas in hydrogen, the electron is in s-orbital.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Because of ionization enthalpy, hydrogen resembles more with halogens, is 520 kJ mol-1, F is 1680 kJ mol-1and that of His 1312 kJ mol-1. Like halogens, it forms a diatomic molecule, combining with elements to form hydrides and a large number of covalent compounds.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
