Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: C
There is no alpha hydrogen atom in the Cannizaro process. Formaldehyde and aromatic aldehydes do not contain alpha hydrogen. It proceeds through the Cannizaro reaction. Formaldehyde is the most reactive of all aldehydes.
New answer posted
4 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: D
Because of the presence of electron withdrawing carbonyl groups, the alpha hydrogen atom in carbonyl compounds is acidic. In nature, hydrogen is very acidic.
Because the cation is released in the form of H+ , the anion created after the loss of the -hydrogen atom is resonance stabilised.
New answer posted
4 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: E
The two reactions produce different products for different reasons. One involves the oxidation of aldehydes, while the other involves the reduction of carboxylic acids. Aldehydes are easily oxidised by mild oxidising agents, hence compounds containing - CHO are rapidly oxidised.
New answer posted
4 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: A
The smallest aldehyde is formaldehyde. It features a triangular planar shape with two bond pairs and no lone pairings. C and O share one pair of electrons in their double bond. As a result, the C atom is sp2 hybridised. As a result, both Assertion and Reason are true.
New answer posted
4 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: A and B
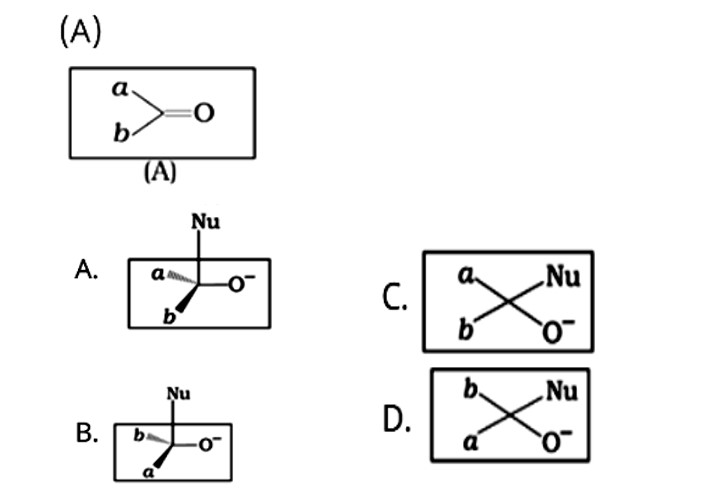
The given compound is a planar molecule with sp2 hybridised carbon. Carbon atoms are attacked by nucleophiles. If the nucleophile approaches from the front, the A and B molecules shift to the front and back positions, respectively, and the carbon becomes tetrahedral. Another alternative is that A and B molecules will be located above and below the plane, respectively. As a result, the choices C and D aren't represented as planar molecules.
New answer posted
4 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: A and B
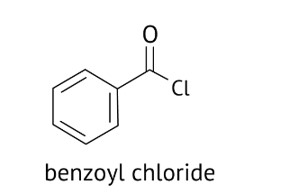
A: Benzophenone can be obtained by Friedel-craft acylation reaction
B: Benzophenone can also be obtained by the reaction between benzoyl chloride and diphenyl cadmium.
New answer posted
4 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: A and C
Grignard Reaction is the addition of an organomagnesium halide to form a tertiary or secondary alcohol.
Canizzaro's Reaction involves the base induced disproportionation of two molecules. Aldol Condensation is an organic reaction when an enolate ion reacts with a carbonyl compound to form beta hydroxy ketone with dehydration to give a conjugated enone.
The HVZ reaction involves alpha bromination of carboxylic acids and this reacts with chlorine or bromine in presence of a small amount of red phosphorus to give alpha halo carboxylic acids.
New answer posted
4 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Solution: (b, d)
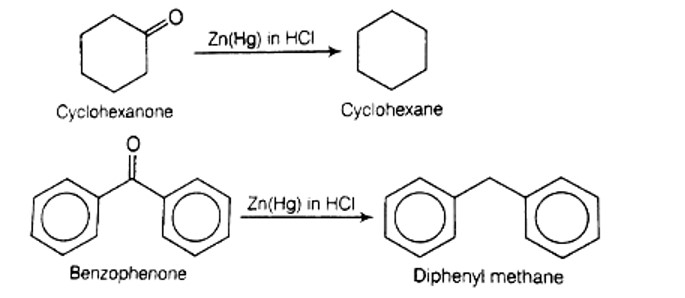
Clemmensen reduction is used to convert cyclohexanone into cyclohexane and benzophenone into diphenyl methane .
New answer posted
4 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: B and C
New answer posted
4 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: B and D
Consider the following product CH3 -CHO C and O are broken into positive and negative bonds. C should have one condition: hydrogen must be available; if it isn't, aldol condensation will not occur. When two products are mixed with the availability of - hydrogen, the positive and negative bonds between carbon and hydrogen are exchanged, and water (H20) is eliminated, resulting in aldol condensation.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers