Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
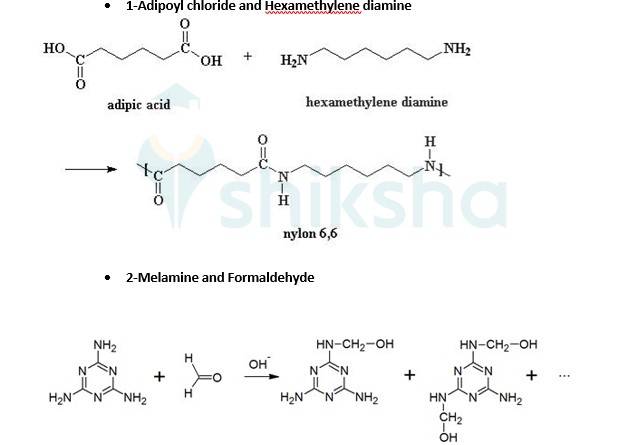
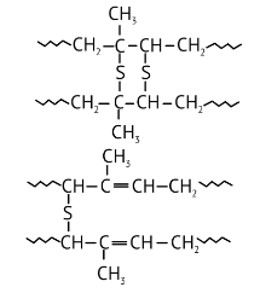
Ans: While manufacturing tyre rubber, 5 % sulfur is used as a crosslinking agent. A natural linear polymer of 2-methyl-1, 3-butadiene becomes hard on treatment with sulfur and forms vulcanised rubber. The structure of the product formed is,
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Thermoplastic polymer
Thermoplastic polymers are linear or slight branched-chain polymers. In this type of polymer, the strong intermolecular force of attraction is present between elastomers and fibers. This type of polymer softens on heating and hardens on cooling. For example, polythene, polystyrene and polyvinyl.
Thermosetting polymer
Thermosetting polymers are cross-linked polymers. These polymers on heating undergo cross-linking in molds. They become infusible on heating so, cannot be reused again. For example, Bakelite and urea-formaldehyde resins.
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:

New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
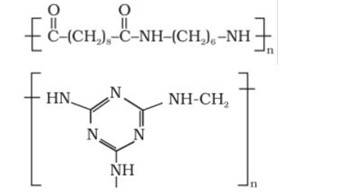
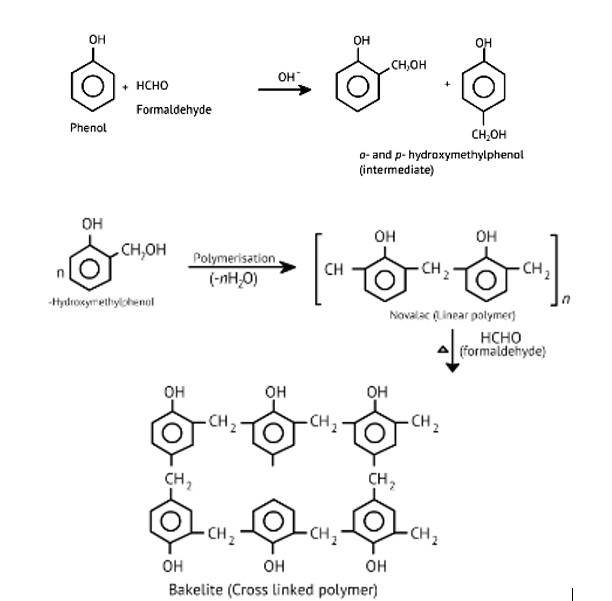
The oldest synthetic polymers are phenol-formaldehyde polymers. These are made by combining phenol and formaldehyde in the presence of either an acid or a base catalyst. The reaction begins with the formation of o-and/or p hydroxymethyl phenol derivatives, which then react with phenol to form compounds with shaving rings connected by –CH2 groups. The first product could be a linear product, such as Novolac.
When heated with formaldehyde, Novolac crosslinks to form Bakelite, an infusible solid mass.
It is used to make combs, phonograph records, electrical switches, and
New answer posted
4 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
In rubber, the polymer chain are held together by the weakest force of attraction. While, in plastic, the strong intermolecular force of attraction is present between elastomers and fibres.
In rubber, a few crosslinks are introduced between the chain, while plastics are linear or slightly branched molecules which gets soft on heating and hard on cooling.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers