Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
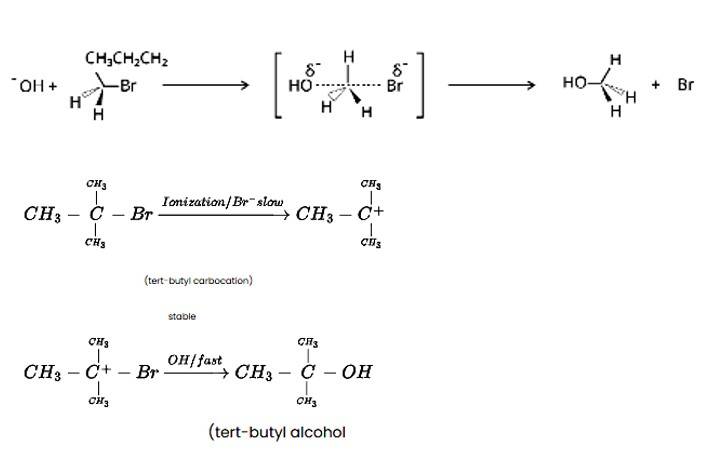
Tert-butyl bromide is substituted via the SN1 process because it may produce a stable carbocation in the first step after the halide group is cleaved. The nucleophile OH - interacts with the carbocation next. The primary halide n-butylbromide, on the other hand, is unable to create a stable carbocation, thus it undergoes the SN2 process, which is a one-step substitution involving OH - attack and concomitant X - leaving to generate n-butyl alcohol.
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
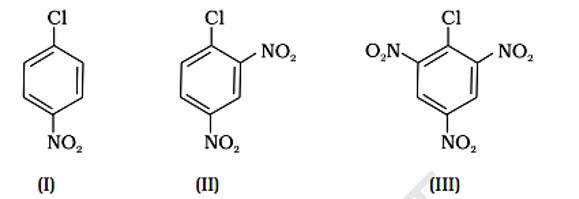
Because of resonance stabilisation, aryl halides are less reactive to nucleophilic substitution. The inclusion of a -NO2, an electron-withdrawing group in the ortho or para position, enhances the aryl halide's sensitivity to substitution. The more electron withdrawing groups there are in the aryl halide, the more reactive it is. The decreasing sequence of reactivity, according to this theory, is III > II > I.
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Nucleophilic substitution and elimination (β-elimination) reactions are both possible with alkyl halides.
Although, with the appropriate reaction conditions and reagent selection, a specific product can be produced. In most cases, the elimination reaction is best suited to strong and bigger bases, as well as high temperatures. The substitution reaction, on the other hand, is best for weaker and smaller bases at lower temperatures.
CH3CH2Br+alc.KOH![]() KCH2=CH2 + HBr
KCH2=CH2 + HBr
CH3CH2Br + aqKOH→CH3CH2OH + KBr
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The IUPAC names for DDT and benzene hexachloride are 2, 2-bis (4-chlorophenyl)-1, 1-trichloroethane and 1, 2, 3, 4, 5, 6-hexachlorocyclohexane, respectively.
They are poisonous and non-bio digestible at the same time. They are fat soluble, and their concentration in the food chain continues to rise. As a result, they are prohibited in India and other nations.
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Diphenyls, such as p, p-dichlorodiphenyltrichloroethane (DDT), which have been employed as organic pesticides, are a possible hazard to the environment since it was shown to have significant toxicity towards fish during its early years of widespread use, and many insects acquired resistance to it. The issue was exacerbated by DDT's chemical persistence and fat solubility. In terms of chemistry, DDT is not metabolised or solubilized well by mammals. The fatty tissues are where it is deposited and kept. DDT builds up within the animal over time if it is consumed at a con
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
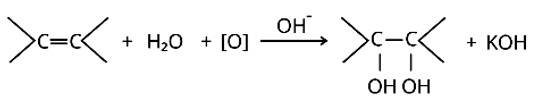
The bromine water test and Bayer's test are two methods for detecting the presence of an unsaturated double bond in an organic molecule.
Bromine is added to the carbon atoms across the double bond in the bromine water test when the molecule is introduced to bromine water. Bromine water changes colour from orange to brown to colourless at the end of the process.
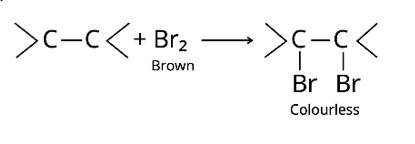
The alkaline KMnO4 is employed in Bayer's test to detect unsaturated carbon with a double bond. The chemical produces [O], which hydrolyzes the carbons across the double bond, resulting in a purple-to-colorless s
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The SN1 mechanism advances through the production of carbocation. The breakage of the C-halogen bond occurs during this phase. The solution of the halide ions is done with the protons of the protic solvent as a result. In this approach, polar solvents aid in the ionisation process by solvating the ions and stabilising them.
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
RMgX+H2O→RH+Mg (OH)X
Because the Grignard reagents are very reactive in nature, even residues of moisture must be avoided. Even minute amounts of water can cause these reagents to generate hydrocarbons.
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In nature, allyl chloride becomes very reactive due to the production of carbocation, which is extremely stable due to resonance. Due to carbocation, there will be no such stability in the case of n-propyl chloride.
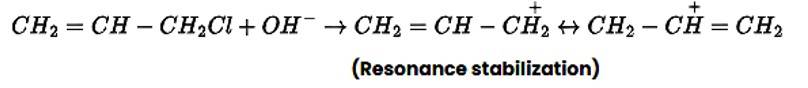
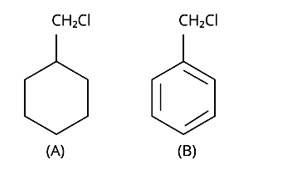
New answer posted
4 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
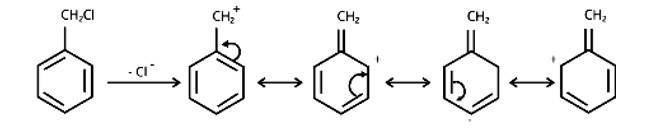
Due to the resonance stabilisation of the benzyl ring, compound (B) would conduct the SN1 reaction faster than compound (A). The carbocation C6H5CH2Cl+ is very stable when the -Cl from the ring is cleaved, and it is driven to carry out the SN1 reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers