P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
New answer posted
4 months agoContributor-Level 10
The inertness of
New answer posted
4 months agoContributor-Level 10
Reducing character increases from NH3 to BiH3.
Group 15 oxides of type E2O3 and E2O5 are not always basic.
New answer posted
4 months agoContributor-Level 10
(2) Cl is in its the highest oxidation state (+7). It cannot be further oxidised
New answer posted
4 months agoContributor-Level 10
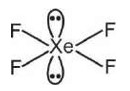
XeF2 : Hybrid orbital = s bond + L.P
= 2 + 3
= 5 = sp3d
Shape ->linear
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers