Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Misch metal consists of Lanthanide metal (? 95%) and iron (? 5%) and traces of S, C, Ca and Al.
New answer posted
4 months agoContributor-Level 10
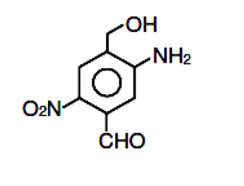
The chemical structure shown is 5-Amino-4- (hydroxymethyl)-2-nitrobenzene carbaldehyde.
New answer posted
4 months agoContributor-Level 10
(i) Ca (OH)? is used in white wash.
(ii) Plaster of paris is used in making of molds for plaster statues.
(iii) NaCl is used in preparation of washing soda.
(iv) A suspension of Mg (OH)? in water is used in medicine as an antacid under name of milk of magnesia.
New answer posted
4 months agoContributor-Level 10
(I) Lucas reagent → Only ZnCl? /Conc. HCl
(II) Dumas method → CuO/Δ
(III) Kjeldahl's method → Conc. H? SO? /Δ
(IV) Heinsberg reagent → C? H? SO? Cl/ aq. NaOH
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
In the ccp lattice of oxide ions effective number of O? ² ions = 8 *? + 6 * ½ = 4
In the ccp lattice,
No. of octahedral voids = 4
No. of tetrahedral voids = 8
Given M? atoms occupies 50% of octahedral voids and M? atoms occupies 12.5 of tetrahedral voids
No. of M? metal atoms = 4 *? /? = 2
No. of M? metal atoms = 8 * ¹²·? /? = 1
∴ Formula of the compound = (M? )? (M? )O?
∴ Oxidation states of metals M? & M? respectively are +2 and +4 .
New answer posted
4 months agoBeginner-Level 5
The metal-carbon bonds (M-C) in metal carbonyls are due to the synergic interaction between the metal and carbonyl group. There are two types of bonding between metal and carbonyl group.
? -bond in Metal Carbonyls: The Carbonyl (CO) ligand donates a lone pair of electrons to the metal center to fill its empty d-filled orbital. This electron density donation is called? -donation.
? -back bond in Metal Carbonyls: This is the case of back bonding. The already filled d-orbitals return the electron density into the empty? * (antibonding) orbital of CO. This electron density donation by the metal is called? -back donation.
Both these comb
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers