Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
Covalent solids have high melting point due to strong covalent bonds, also they are insulator in both solid as well as in molten state.
New answer posted
3 months agoContributor-Level 9
In basic medium H? O? can behaves as both oxidizing and reducing agent, so it can oxidize Mn²? to Mn? and reduce I? to I?
New answer posted
3 months agoContributor-Level 9
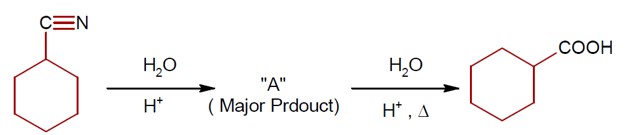
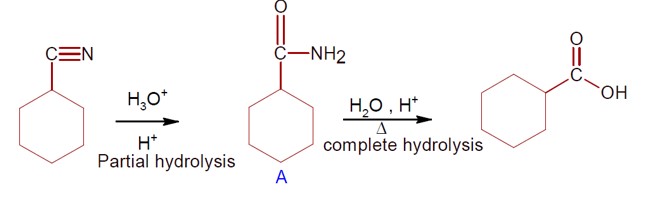
Both C? H? OH and AgCN can generate nucleophile.
KCN generate nitrile as nucleophile while AgCN generate isonitrile as nucleophile in nucleophilic substitution reaction.
New answer posted
3 months agoContributor-Level 9
Given K_f = 1.85 K kg mol? ¹ for a solution with molality of 2 m.
ΔT_f = I * K_f * m
3.885 = I * 1.85 * 2
The van't Hoff factor, I = 1.05.
i = 1 + (n-1)α. For an electrolyte dissociating into 2 ions, n=2.
1.05 = 1 + (2-1)α.
The degree of dissociation, α = 0.05 or 50 * 10? ³.
New answer posted
3 months agoContributor-Level 9
For an acidic buffer solution, pH = pKa + log ( [Base]/ [Acid]).
Given pH = 5.74 and pKa = 4.74.
5.74 = 4.74 + log ( [Base]/1).
1 = log ( [Base]).
[Base] = 10M.
New answer posted
4 months agoContributor-Level 9
o Let the number of atoms of element A be N.
o Tetrahedral voids = 2N.
o Atoms of element M = (2/3) * 2N = 4/3 N.
o The ratio M : A is (4/3 N) : N, which simplifies to 4:3.
o So, the formula of the compound is M? A?
New question posted
4 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers