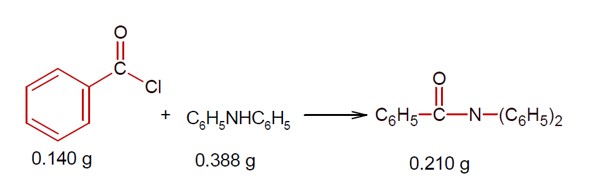
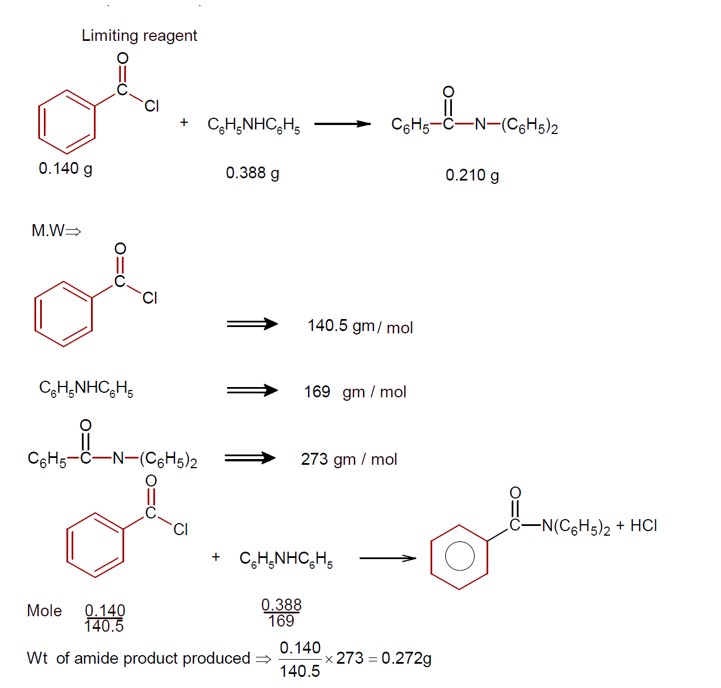
Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
For the dissociation of K? [Fe (CN)? ]? 4K? + [Fe (CN)? ]? , the number of ions produced (n) is 5.
The degree of dissociation (α) is related to the van't Hoff factor (i) by α = (i-1)/ (n-1).
Given α = 0.4: 0.4 = (i - 1) / (5 - 1) => 1.6 = I - 1 => I = 2.6
Using the depression in freezing point formula (ΔTf = i·Kf·m), and equating the ΔTf for two different solutions:
ΔTf (K? [Fe (CN)? ]) = ΔTf (A)
i? ·Kf·m? = i? ·Kf·m?
(2.6) * [ (18.1 / M) / (100-18.1)/1000] = (1) * [ (w? /M? ) / (W? /1000)]
The problem simplifies to finding the molar mass M of solute A:
2.6 * [18.1 / (M * 81.9)] * 1000 = [w? /M? ] * [1000/W? ]
Assuming the secon
New answer posted
4 months agoContributor-Level 10
3CaO + 2Al → 3Ca + Al? O?
ΔH? = ΣΔH? (Products) - ΣΔH? (Reactants)
ΔH? = [ (0) + (-1675) ] - [ (3 * -635) + (0) ]
ΔH? = -1675 - (-1905) = -1675 + 1905 = 230 kJ
Ans = 230
New answer posted
4 months agoContributor-Level 10
Cr? O? ²? + Fe²? - (H? )-> Cr³? + Fe³?
(n=6) (n=1)
Meq Cr? O? ²? = Meq Fe²?
20 * 0.03 * 6 = 15 * M * 1
M = (20 * 0.03 * 6) / 15 = 0.24 = 24 * 10? ²
Ans = 24
New answer posted
4 months agoContributor-Level 10
P? + 3NaOH + 3H? O → PH? + 3NaH? PO? (A)
Here, NaH? PO? is a reducing agent.
NaH? PO? + AgNO? + H? O → Ag + HNO? + NaH? PO?
Equivalents of NaH? PO? = equivalents of Ag (n-factor of NaH? PO? = 4)
1 mole * 4 = n moles of Ag * 1
So, moles of Ag = 4.
New answer posted
4 months agoContributor-Level 10
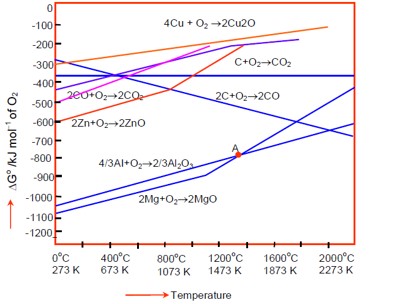
At the point of intersection in an Ellingham diagram? G for two processes becomes equal, so? G for the reduction becomes zero. A sudden increase in the slope indicates a change in the state of the metal oxide, i.e., from solid to liquid or liquid to vapor.
New answer posted
4 months agoContributor-Level 10
In the ammonolysis process, bond cleavage is carried out in the presence of NH? When a halide compound is treated with NH? , the halide ion (X? ) is substituted by an amino group (NH? ) in a nucleophilic substitution reaction.
New answer posted
4 months agoContributor-Level 10
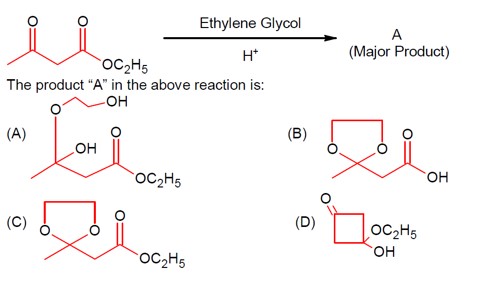
A more reactive carbonyl, i.e., a ketone, is masked with ethylene glycol first in an acidic medium.
New answer posted
4 months agoContributor-Level 10
Allosteric inhibitors are a type of drug-enzyme inhibitor that binds to a site other than the active site of the enzyme, causing a conformational change that affects the active site. The statement in the document incorrectly states they bind at the active site due to steric factors.
New answer posted
4 months agoContributor-Level 10
A divalent metal ion with an atomic number (Z) of 25 is Mn²?
Mn²? (Z=25) has the electronic configuration: 3d? 4s? (n=5, five unpaired electrons).
The magnetic moment (μ) is calculated as: μ = √ [n (n+2)] = √ [5 (5+2)] = √35 = 5.92 BM.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers