Physics NCERT Exemplar Solutions Class 11th Chapter Twelve
Get insights from 37 questions on Physics NCERT Exemplar Solutions Class 11th Chapter Twelve, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics NCERT Exemplar Solutions Class 11th Chapter Twelve
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
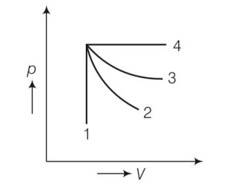
(c) For the curve 1 volume is constant so it is isochoric process. But in curve 2 and 3 curve 2 is steeper so 2 is adiabatic and 3 is isothermal.
New answer posted
7 months agoContributor-Level 10
process 1 isochoric and process 2 is isothermal .
Since, work done = area under P-V curve . here area under the pV curve 1 is more . so work done is more when the gas expands in isochoric process.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Coefficient of
T1= 27+273=300K
Coefficient of performance
1500-5T2=T2
6T2=1500
T2= 250K
T2= 250-273=-23oC
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Temperature of the source is 270C
T1= 27+273= 300K
T2= -3+273= 270K
Efficiency of heat engine = 1-T2/T1= 1-270/300=1/10
Efficiency of refrigerator is 50% of a perfect engine
= 0.5 = 1/20
Coefficient of performance of the refrigerator
=
Q2= =19W
= 19 1KW=19KW= 19kJ/s
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
For adiabatic change process we know
P1V1y= P2V2y
P (V+ )y = (P+ )Vy
PVy (1+ ) y=p (1+ )Vy
PVy (1+ ) PVy (1+ )
Y
dV=
hence work done increasing the pressure from P1 to P2
W=
=
W=
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Height of stairs h= 10m
Energy produced by burning 1 kg of fat = 7000Kcal
Energy produced by burning 5kg of fat = 5
Energy utilised in going up and down one time
= mgh + =
=
= 9000J= 9000/4.2=3000/1.4cal
Number of times, the person has to go up and down the stairs
= = 16.3 times
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Temperature of the source T1= 500K and sink T2= 300K
Work done W= 1000J
Efficiency of Carnot engine = 1-T2/T1= 1-300/500= 200/500= 2/5
Efficiency = W/Q1
So Q1= W/efficiency = 1000
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
During driving temperature of the gas increases while volume remains constant. So according charle's law, at constant volume V.
Pressure is directly proportional to temperature. Therefore pressure of gas increases.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Yes during adiabatic compression the temperature of a gas increases while no heat is
In adiabatic compression dQ=0
From the first law of thermodynamics dU= dQ-dW
dU=-dW
in compression work is done on the gas i.e work done is negative
dU=positive
hence internal energy of the gas increases due to which its temperature increases.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
If a refrigerator's doors is kept open, then room will become hot, because amount of heat removed would be less than the amount of heat released in the room.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers