Physics Ncert Solutions Class 12th
Get insights from 1.2k questions on Physics Ncert Solutions Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Ncert Solutions Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
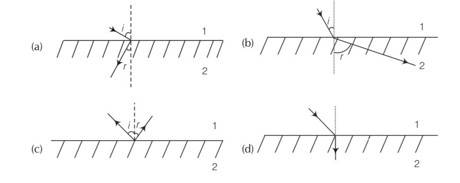
(a, b, c) When immersed object is seen from close to the edge of the trough the object looks distorted because the apparent depth of the points close to the edge are nearer the surface of the water compared to the points away from the edge.
The angle subtended by the image of the object at the eye is smaller than the actual angle subtended by the object in air and some of the points of the object far away from the edge may not be visible because of total internal reflection.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a) The negative refractive index metamaterials are those in which incident ray from air (Medium 1) to them refract or bend differently to that of positive refractive index medium.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(d) The speed of the image of the car would appear to increase as the distance between the cars decreases.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
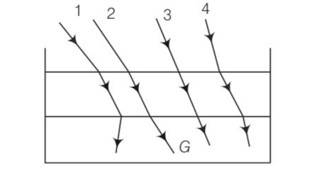
Here, light ray goes from (optically) rarer medium air to optically denser terpentine, then it bends towards the normal i.e., i>r whereas when it goes from to optically denser medium terpentine to rarer medium water. then it bends away the normal i.e., i
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
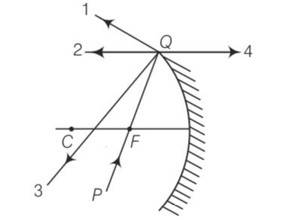
(b) The PQ ray of light passes through focus F and incident on the concave mirror, after reflection, should become parallel to the principal axis and shown by ray-2 in the figure.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) The phenomenon involved in the reflection of radio waves by ionosphere is similar to total internal reflection of light in air during a mirage i.e., angle of incidence is greater than critical angle.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) R= 20cm, = 1.5, f= = =40cm so lens acts as convex lens.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) According to VIBGYOR, among all given sources of light, the blue light have the smallest wavelength. According to Cauchy relationship, smaller the wavelength higher the refractive index and consequently smaller the critical angle. So, corresponding to blue colour, the critical angle is least which facilitates total internal reflection for the beam of blue light. The beam of green light would also undergo total internal reflection.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) A passenger in an aeroplane may see a primary and a secondary rainbow like concentric circles.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers