Physics
Get insights from 5.6k questions on Physics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- refractive index = 1.38 refractive index = 1.5
0
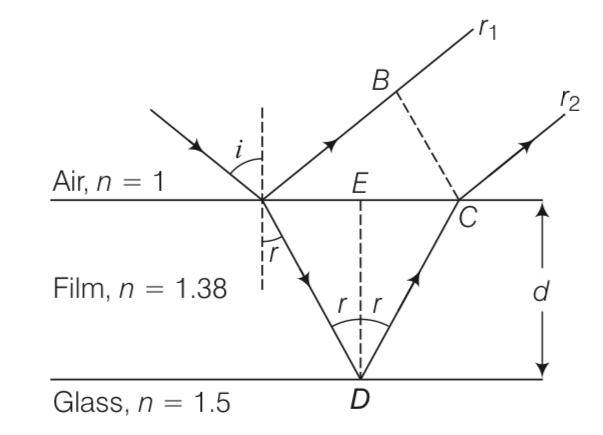
Consider a ray incident at an angle i. A part of this ray is reflected from the air-film interface And apart refracted inside.
This is partly reflected at the film-glass interface and a part transmitted. A part of the
reflected ray is reflected at the film-air interface and a part transmitted as r2 parallel to r 1. Of course successive reflections and transmissions will keep on decreasing the amplitude of the wave. Hence, rays r 1 and r2 shall dominate the behaviour. If incident light is to be transmitted thro
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation-All points with the same optical path length must have the same phase.
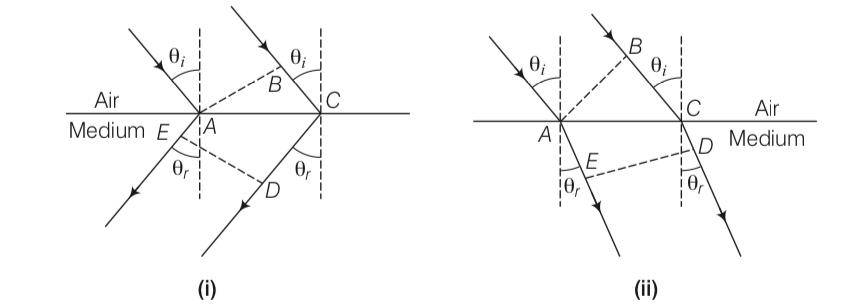
So – =BC-–
BC= (CD-AE)
BC>0, si must be greater than AD
But in other figure
–
So BC= –
But clearly here BE is less than zero
To proving snells law we know that
BC=ACsin and CD-AE=ACsin
So n= sini/sinr
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
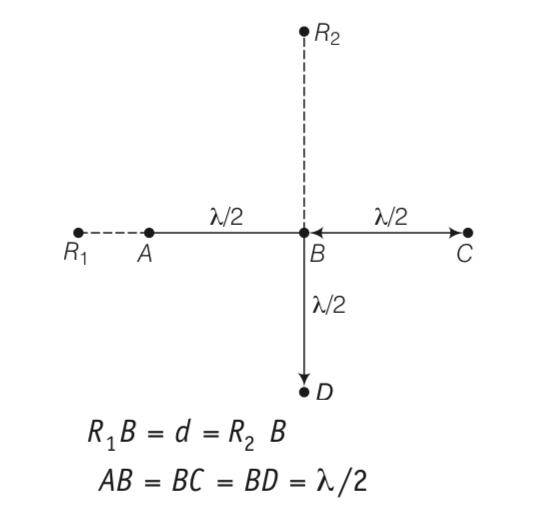
Explanation-consider the disturbance at the receiver R1 which is at a distance d from B
YA= acos(wt) and path difference is hence phase difference is .
Thus the wave R1 because of B
YB= acos(wt- )= - acoswt here path difference is and hence phase difference is
Thus R1 because of C
Yc= acos(wt-2 )= acoswt
(i)let the signal picked up at R2 from B be YB= a1cos(wt)
The path difference between signal at D and that B is
YD= -a1cos(wt)
The path difference between signal at A and that atB is
-d = d( -d =
therefore path difference os 0
A=a1co
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
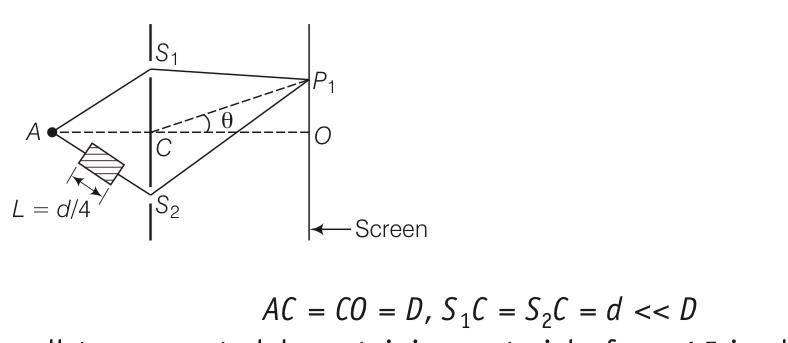
Explanation- as the refractive index of the class , the path difference will be calculated as =2dsin +( )L
For principal maxima ,(path difference is zero)
2dsin 0+( )L=0
Sin 0= - =
Sin 0=-1/16
OP=Dtan 0= Dsin 0=-D/16
For pat h difference
2dsin 1+0.5L=
Sin 1= =
= = 1/4 -1/16
So two possible values and- =
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- when polariser is not used
A=Aperp+A
letA1= asinwt and A2=asin(wt+ )
now superposition principle for perpendicular polariser
AR= asinwt+ asin(wt+ )
AR=a(2cos sin(wt+ ))
AR=2acos sin(wt+ )
This eqn is also same for parallel polariser
AR=2acos sin(wt+ )
And we know that intensity is directly proportional to square of amplitude
(AR)2= (Aperp)2+(A)2
So resultant intensity is
I=4(a)2cos2 dt + 4(a)2cos2 dt
I= 8(a)2cos2 (1/2) &nb
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (c, d)
Explanation- The simple Bohr model is not applicable to He4 atom because He4 has one more electron and electrons are not subject to central forces.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b, d)
Explanation- when a radiation of energy fall on it some atoms would be excited but not all would be excite also no atom will go to 3 level some go to 2 level also but not all excite to 2 level. So by considering these facts we can say b and d option are correct
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (b, d)
Explantion- Balmer series for the H-atom can be observed if we measure the frequencies of light emitted due to transitions between higher excited states and the first excited state and as a sequence of frequencies with the higher frequencies getting closely packed.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (a, b)
Explantion- The Bohr model for the spectra of a H-atom will not be applicable to hydrogen in the Molecular form. And also, it will not be applicable as it is for a He-atom.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer- (a, b)
Explantion- When beam of free electrons is aiming towards free protons. Then, they scatter but an electron and a proton cannot combine to produce a H-atom because of energy conservation and without simultaneously releasing energy in the form of radiation.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers