Polymers
Get insights from 155 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
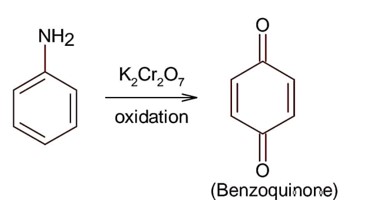
Only 1° aromatic amines give stable diazonium salt on reaction with nitrous acid
Here 1° aromatic amine is 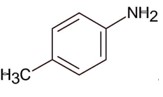
New answer posted
5 months agoContributor-Level 10
Given let α, β be the roots of the equation x2 + bx + c = 0
So,
Also x2 + bx + c = (x - α) (x - β)
=
New answer posted
5 months agoContributor-Level 9
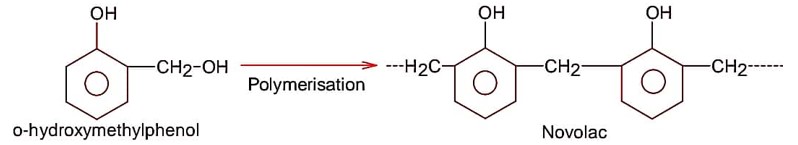
Monomer of novolac is
Molecular mass of monomer unit
= 12 * 7 + 1 * 8 + 16 * 2
= 84 + 8 + 32
= 124 g
Molecular mass of polymer = 963 g
(n – 1) unit of H2O is removed during formation
106n = 945
New answer posted
5 months agoContributor-Level 9
FeCl3. 3H2O i.e. [Fe (H2O)3], K3 [Fe (CN]6 and [Co (NH3)6]Cl3 the last two complex are inner-orbital complex due to presence of strong ligand.
Because CN- is strong ligand
More smaller value of absorbed
= 1.732 B.M
2
New answer posted
5 months agoContributor-Level 10
Bakelite formed by the condensation reaction of phenol with formaldehyde and it is a thermosetting polymer/ plastics.
New answer posted
5 months agoContributor-Level 10
Monomer of novolac is
Molecular mass of monomer unit
= 12 * 7 + 1 * 8 + 16 * 2
= 84 + 8 + 32
= 124 g
Molecular mass of polymer = 963 g
n * mass of monomer = mass of polymer + (n – 1) * 18
(n – 1) unit of H2O is removed during formation
106n = 945
New answer posted
5 months agoContributor-Level 10
Bakelite used for making electrical switches, Glyptal used for paints and lacquers, PVC used for water pipe and polystyrene used for Television cabinets.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers