Polymers
Get insights from 155 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
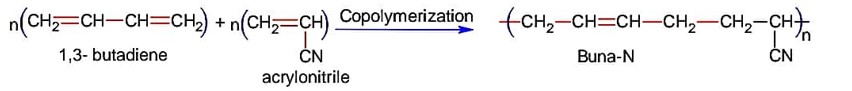
Buna – N is synthetic rubber which can be stretched and retains its original status on releasing the force.
New answer posted
5 months agoContributor-Level 10
Vulcanization of rubber is carried out by heating a mixture of isoprene and sulphur.
New answer posted
5 months agoContributor-Level 10
Copolymer is the polymer formed by two or more monomers having multiple bonds.
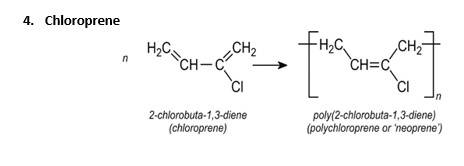
Buna – S, PHBV and butadiene – styrene are copolymers. Neoprene is homopolymer.
New answer posted
5 months agoContributor-Level 10
No. of Amino acid units are 4 and
No. of peptide bonds are 3
In tetrapeptide
New answer posted
6 months agoContributor-Level 10
Buna-N is obtained by copolymerization of 1, 3-butadiene and acrylonitrile,
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(iv) Assertion is correct statement and reason is wrong statement. (v) Assertion is wrong statement and reason is correct statement.
Explanation: Most of the Synthetic polymers are not biodegradable. A large number of the polymers are resistant to the degradation process of the environment and therefore, are responsible for the accumlation of polymeric solid waste material.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) Assertion and reason both are correct statements but reason does not explain assertion.
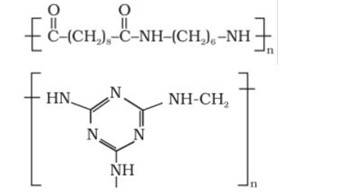
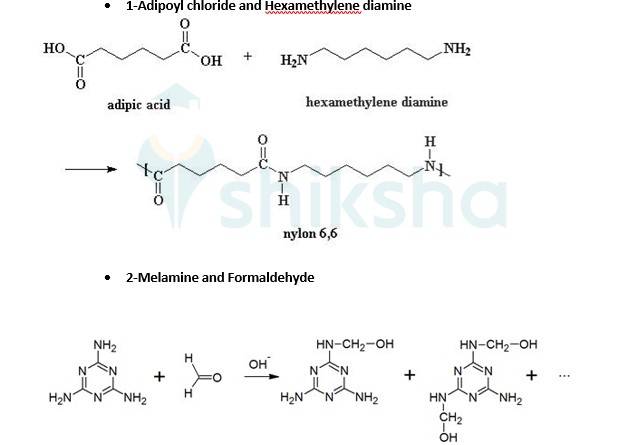
Explanation: Thermosetting polymers are cross linked polymers. They are highly branched polymers with high molecular masses. These polymers on heating undergo cross linking in moulds. They become infusible on heating.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(v) Assertion is wrong statement and reason is correct statement.
Explanation: Synthetic rubber is a type of rubber polymer that are capable of getting stretched to twice of its length. They are homopolymers of either 1,3-butadiene derivatives or copolymers of butadiene. Polychloroprene or neoprene is a type of synthetic rubber.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers