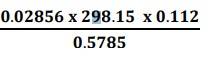Some Basic Concepts of Chemistry
Get insights from 131 questions on Some Basic Concepts of Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Some Basic Concepts of Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
1.34. (i) 1 mole (44 g) of CO2 will have 12 g carbon.
So, 3.38 g of CO2 will have carbon = 12g/44g * 3.38
= 0.9217 g
18 g of water will have 2 g of hydrogen.
So, 0.690 g of water contain hydrogen = 2g/18g * 0.6902g
= 0.0767 g
Since carbon and hydrogen are the only constituents of the compound, the total mass of the compound is:
= 0.9217 g + 0.0767 g
=0.9984 g
So, the percentage of Carbon in the compound = 0.9217/0.9984 * 100 = 92.32%
Now, percentage of Hydrogen in the compound = 0.0767/0.9984 * 100 = 7.68%
Moles of carbon in the compound = 92.32/12=7.69
Moles of hydrogen in the compound = 7.68/1=7.68
Since, we ha
New answer posted
9 months agoContributor-Level 10
1.33. (i) 52 moles of Ar
Ans:1 mole of Ar = 6.022 * 1023 atoms of Ar
Therefore, 52 mole of Ar = 52 * 6.022 * 1023 atoms of Ar= 3.131 * 1025 atoms of Ar
(ii) 52 u of He
Ans:1 atom of He = 4u of the He
Or, 4 u of He = 1 atom of He
So, 52 u of He = 52/4 atom of He = 13 atoms of He.
(iii) 52 g of He
Ans:4g of He = 6.022 * 1023 atoms of He
So, 52g of He = 6.022 * 1023 * 52/4 atoms of He
= 7.8286 * 1024 atoms of He
New answer posted
9 months agoContributor-Level 10
1.32. Molar mass of argon is
= [ (35.96755 * 0.337/100)+ (37.96272 * 0.063/100)+ (39.9624 * 99.60/100)]g mol-l
= [0.121+0.024+39.802] g mol-l
= 39.947 g mol-l
So, the molar mass of argon is 39.947 g/ mol.
New answer posted
9 months agoContributor-Level 10
1.31. First we need to find the least precise number to find the significant figures.
(i) Least precise number of the calculation![]() is 0.112
is 0.112
Therefore, number of significant figures in the answer = Number of significant figures in the least precise number, i.e. 3
(ii) Least precise number of calculations = 5.364
Therefore, number of significant figures in the answer will be = Number of significant figures in 5.364 = 4
(iii) 0.0125+0.7864+0.0215
Since the least number of decimal places in each term is four, the number of significant figures in the answer will also be 4.
New answer posted
9 months agoContributor-Level 10
1.30. 1 mol of 12C atoms = 6.02 x 1023 atoms = 12 g
Or, 6.02 x 1023 atoms of 12C have mass = 12 g
Therefore, 1 atom of 12C will have mass = 12 x 1/6.02 x 1023 = 1.9927 x 10-23 g
New answer posted
9 months agoContributor-Level 10
1.29. According to the formula of mole fraction,
X (C2H5OH) = 0.040 = n (ethanol) / [n (ethanol) + n (water)]
Now, n (water) = 1000g/18g mol-1 = 55.55 moles [? Density of water=1kg m-3]
Therefore, 0.040 = n (ethanol) / [n (ethanol) + 55.55]
i.e., 0.04 x n (ethanol) + 2.222 = n (ethanol)
i.e., 2.222 = [1- 0.04] n (ethanol)
i.e., n (ethanol) = 2.222 / 0.96 = 2.314 M
New answer posted
9 months agoContributor-Level 10
1.28. The number of atoms in each of the given elements are calculated as below:
(i) 1 g Au = 1 / 197 mol = 1/ 197 x 6.02 x 1023 atoms
(ii) 1 g Na = 1/ 23 mol = 1/ 23 x 6.02 x 1023 atoms
(iii) 1 g Li = 1/ 7 mol = 1 / 7 x 6.02 x 1023 atoms
(iv) 1 g of Cl2 = 1/ 71 mol = 1/ 71 x 6.02 x 1023 atoms
Therefore, since the denominator is the smallest in case of Li, 1 g Li has the largest number of atoms
New answer posted
9 months agoContributor-Level 10
1.27. (i) 28.7 pm = 28.7 x 10-12 m = 2.87 x 10-11 m
(ii) 15.15 µs = 15.15 x 10-6 s = 1.515 x 10-5 s
(iii) 25365 mg = 25365 mg x 10-6 kg = 2.5365 x 10-2 kg
New answer posted
9 months agoContributor-Level 10
1.26. H2 and O2 react according to the equation
2H2 (g) + O2 (g) ——>2H2O (g)
Thus, 2 volumes of H2 react with 1 volume of O2 to produce 2 volumes of water vapour. Hence, 10 volumes of H2 will react completely with 5 volumes of O2 to produce 10 volumes of water vapour.
New answer posted
9 months agoContributor-Level 10
1.25. Molar mass of Na2CO3= (2 x 23) + 12 + (3 x 16) = 106g mol-1
0.50 mol Na2CO3 means 0.50 x 106 g = 53 g of Na2CO3
0.50 M Na2CO3 means 0.50 mol per volume in litre,
i.e., half of 106 g Na2CO3 is present in 1 L solution.
i.e.,53 g Na2CO3 is present in 1 L of the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers