Some Basic Concepts of Chemistry
Get insights from 131 questions on Some Basic Concepts of Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Some Basic Concepts of Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
1.14. S.I. unit of mass is kilogram (kg).
It is defined as the mass of platinum-iridium block stored at international bureau of weights and measures in France.
New answer posted
9 months agoContributor-Level 10
1.13. Pressure= Force/Area
But weight = m x g, where m = mass (in kg) and g = 9.8 m/s2
Therefore, Pressure = 1034 g cm-2 x 9.8 m/s2
= 1034 x 10-3 kg x (100 m)2 x 9.8 m/s2
= 101332 Pa
= 1.01332 x 105 Pa
New answer posted
9 months agoContributor-Level 10
1.12. Density of methanol = 0.793 kg/L, molar mass of methanol (CH3OH) = 32g/mol = 0.032 kg/mol
V1 =? , V2 = 2.5 L, M2 = 0.25 M
We can apply the formula of
M1V1 = M2V2
Or V1 = M2V2/M1
Substituting M1 = density / molar mass, we get
M1 = 0.793/0.032 = 24.78
V1 = 0.25 x 2.5 / 24.78 = 0.02522 L = 25.22 mL
New answer posted
9 months agoContributor-Level 10
1.11. Molar mass of sugar = (12 x 12) + (22 x 1) + (11 x 16) =342 g/mol
No. of moles in 352 g of sugar = 1 mol
No. of moles in 20 g = 20 x 1/352 = 0.0585 mol
Therefore, molar concentration = moles of solute / volume of solution in L = 0.0585 / 2 = 0.0293 mol/L
New answer posted
9 months agoContributor-Level 10
1.10. (i) 1 mole of C2H6 contains 2 moles of carbon atoms
Therefore, no of moles of C atoms in 3 moles of C2H6 = 6 moles
(ii) 1 mole of C2H6 contains 6 moles of hydrogen atoms
Therefore, no of moles of H atoms in 3 moles of C2H6 = 18 moles
(iii) 1 mole of C2H6 contains 6.02 x 1023 molecules
Therefore, 3 moles of C2H6 will contain ethane molecules = 3 x 6.02 x 1023 molecules= 18.06 x 1023 molecules
New answer posted
9 months agoContributor-Level 10
1.9. Average atomic mass = (Fractional abundance of 35Cl x molar mass of 35Cl) + (Fractional abundance of 37Cl x molar mass of 37Cl)
= (75.77/100 x 34.9689) + (24.23/100 x 36.9659)
= 26.4959 + 8.9568
= 35.4527
New answer posted
9 months agoContributor-Level 10
1.7. 1 mole of CuSO4 contains 1 mole (1 g atom) of Cu
Molar mass of CuSO4= 63.5 + 32 + (4 x 16) = 159.5 g mol-1
Thus, Cu that can be obtained from 159.5 g of CuSO4 = 63.5 g
∴ Cu that can be obtained from 100 g of CuSO4 =63.5/159.5 * 100 = 39.81g
New answer posted
9 months agoContributor-Level 10
1.6. A mass percent of 69% means that 100 g of nitric acid solution contains 69 g of nitric acid by mass.
Molar mass of nitric acid HNO3= 1 + 14 + (3x16) = 63 gmol-1
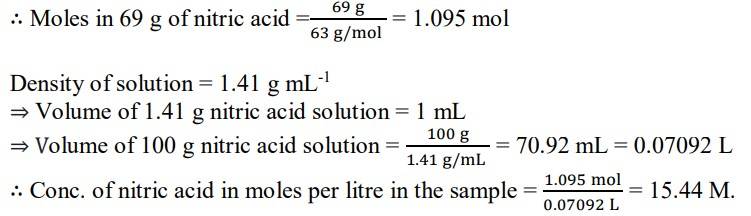
New answer posted
9 months agoContributor-Level 10
1.5
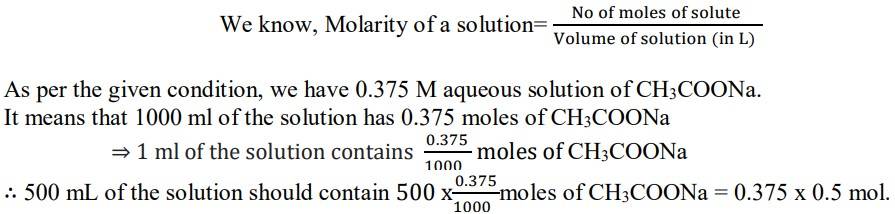
Since the molar mass of sodium acetate is 82.0245 g mol-1.
⇒ 1 mol of CH3COONa has a mass = 82.0245 g
∴Mass of sodium acetate (CH3COONa) required to make 500 ml of 0.375 molar aqueous solution
= 0.375 x 0.5 mol x 82.0245 g mol-1
= 15.3795 g = 15.380 g
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers