Some Basic Concepts of Chemistry
Get insights from 131 questions on Some Basic Concepts of Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Some Basic Concepts of Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
1.4 In order to answer the question, we need to know the balanced equation for the combustion of carbon in dioxygen/air, which can be written as:
C (s) + O2 (g) à CO2 (g)
(i) We can see from the above equation,
1 mole of carbon reacts with 1 mole of oxygen to produce 1 mol of carbon dioxide.
In air, combustion is complete.
Therefore, CO2 produced from combustion of 1 mole of carbon= Molar mass of CO2= 44 g
(ii) As only 16 g of dioxygen is available, it can combine only with 0.5 mole of carbon, i.e., dioxygen is the limiting reactant.
Hence, CO2 produced = 22 g
Here, dioxygen acts as the limiting reagent.
(iii) Here again, dioxygen is
New answer posted
9 months agoContributor-Level 10
1.3 We are given that the percentage of iron by mass is 69.9% and the percentage of oxygen by mass is 30.1%.
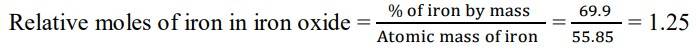
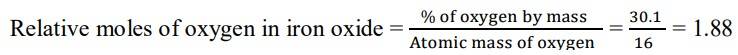
Since we have relative moles of both elements, we can calculate the simpler molar ratio of iron to oxygen
= 1.25: 1.88
Divide by the smaller value to both
= 1.25/1.25: 1.88/1.25
= 1: 1.5
= 2: 3
So, now we can write the empirical formula of iron oxide as Fe2O3.
New answer posted
9 months agoContributor-Level 10
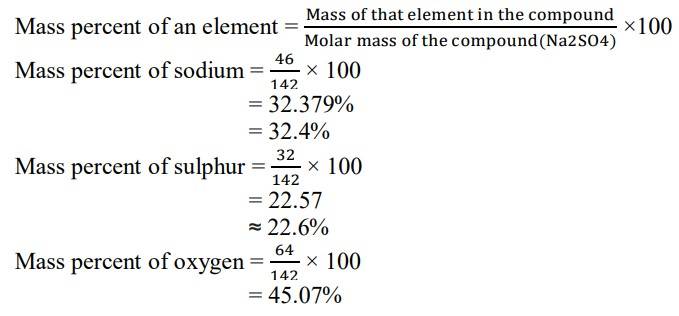
1.2 Molar mass of Na2SO4= (2 x Atomic mass of Sodium) + Atomic mass of Sulphur + (4 x Atomic mass of Oxygen)
= (2x 23) + 32 + (4x 16)
= 46 + 32 + 64
= 142 g/mol
New answer posted
9 months agoContributor-Level 10
1.1. (i) Molecular mass of H2O = (2x Atomic mass of Hydrogen)+ Atomic mass of Oxygen
Atomic mass of Hydrogen = 1.008 amu
Atomic mass of Oxygen = 16.00 amu
So,
Molecular mass of H2O = 2 (1.008 amu) + 16.00 amu =18.016 amu
(ii) Molecular mass of CO2 = Atomic mass of Carbon + (2x Atomic mass of Oxygen)
Atomic mass of Carbon = 12 amu
Atomic mass of Oxygen = 16 amu
So,
Molecular mass of CO2 = 12.01 amu + 2 x 16.00 amu = 44.01 amu
(iii) Molecular mass of CH4 = Atomic mass of Carbon+ (4x Atomic mass of Hydrogen)
Atomic mass of Carbon = 12 amu
Atomic mass of Hydrogen = 1.008 amu
So,
Molecular mass of CH4 = 12.01 amu + 4 (1.008 a
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers
