States of Matter
Get insights from 92 questions on States of Matter, answered by students, alumni, and experts. You may also ask and answer any question you like about States of Matter
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Aluminium is more electropositive than Cr, so it displaced chromium from Cr? O?
Cr? O? + Al - (Δ)-> Al? O? + Cr
New answer posted
5 months agoContributor-Level 10
M = (a³ * d * N_A) / Z = (3.608 * 10? )³ * 8.92 * 6.022 * 10²³) / 4
M = (46.96 * 10? ²? * 8.92 * 6.022 * 10²³) / 4 = 63 g/mole
the closest answer is choice (1)
P = (nRT) / V = (2 * 0.0831 * 300) / 10 = 4.986 bar
New answer posted
5 months agoContributor-Level 10
p? = p? x? is not a correct form of Dalton's law of partial pressures.
New answer posted
5 months agoContributor-Level 10
Mole of CH? = 6.4 / 16 = 0.4 and mole of CO? = 8.8 / 44 = 0.2
Total mole = (0.4 + 0.2) = 0.6 mole of a non-reacting mixture of gas
Using Ideal Gas Law; P = nRT / V
P = (0.6 * 8.314 * 300) / 10 = 149.65 kPa
Ans = 150 (Rounded off)
New answer posted
5 months agoContributor-Level 10
Partial Pressure of O? = K? * solubility (K? = Henry's constant)
Solubility = PO? / K? = 20 / (8.0 * 10? ) = 2.5 * 10? = 25 * 10? M
Ans = 25
New answer posted
5 months agoContributor-Level 9
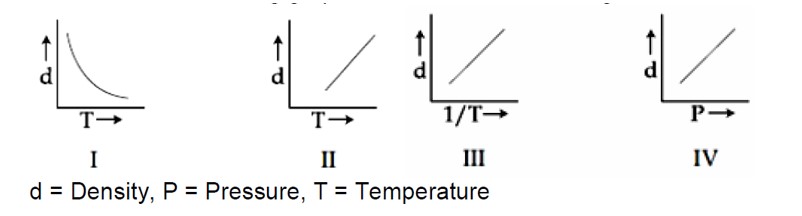
For ideal gas
PM = dRT
d = [PM]/R * 1/T
So graph between dV ST is not straight line
New answer posted
6 months agoContributor-Level 10
Applying :
Assuming the system attains a final temperature of T (Such that 300 < T < 60)
(Heat lost by N2 of container l) = (Heat gained by N2 of container II)
14 (300 – T) = T – 60
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers