The d and f Block Elements
Get insights from 101 questions on The d and f Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The d and f Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
7. (A) 2I- + S2O82- →Fe (III) I2 + 2SO42-
role of fe ions:
2Fe3+ + 2I- → 2Fe2+ + I2
2Fe2+ + S2O82- → 2Fe3+ + 2SO42-
(B) 1. Fine powdered state of Nickel is used in the hydrogenation of oils into fats.
2. Iron is used in the formation of ammonia in Haber's process.
3. In contact process in manufacture of H2SO4, vanadium is used in its oxide form- V2O5.
New answer posted
9 months agoContributor-Level 10
6. Interstitial compounds are formed when small atoms like H, C and N get trapped inside the crystal lattice of transition metals.
Characteristics:
They are hard and rigid in nature having high melting points.
Like pure metals only, they are conductors. They can gain chemical inertness.
New answer posted
9 months agoContributor-Level 10
5. (A) (i) Out of all the elements of the first transition series copper has the highest second ionisation enthalpy.
Electronic configuration of Copper is: 3d104s1
After the Loss of first electron from the 4s copper acquires 3d10 configuration which is stable. Therefore, removal of second electron from the field 3-D orbital is very difficult and requires high amount of energy.
(ii) Among the elements of first transition series zinc has the highest third ionisation enthalpy. Electronic configuration of zinc is: 3d104s2
After the loss of two electrons from 4s orbital, Z and +2 Ion acquires 3d10 fully filled configuration which is highly sta
New answer posted
9 months agoContributor-Level 10
4. (a) As per fajan's rule, smaller the size of ion, greater is its tendency to make covalent bonds as the positively charged cation strongly attract the negatively charged electron cloud.
On moving from La to Lu in the lanthanoid series, the atomic size decreases so the covalent character increases. Hence, La2O3 is ionic and Lu2O3 is covalent.
(b) The stability of Oxo salts is directly proportional to the size of atom, as we move from La to Lu, the size of the atom decreases and hence the stability of oxo salts also decreases.
(c) As we move along the lanthanide series, the atomic size decreases. As a result, the charge/size ratio incr
New answer posted
9 months agoContributor-Level 10
4. As per fajan's rule, smaller the size of ion, greater is its tendency to make covalent bonds as the positively charged cation strongly attract the negatively charged electron cloud.
On moving from La to Lu in the lanthanoid series, the atomic size decreases so the covalent character increases. Hence, La2O3 is ionic and Lu2O3 is covalent.
New answer posted
9 months agoContributor-Level 10
3. (A) = MnO2
(B) = K2MnO4
(C) = KMnO4
(D) = KIO3
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
(A) (B)
3MnO42- + 4H+ → 2MnO4- + MnO2 + 2H2O
(C)
2MnO42- + 2H2O + KI→ 2MnO2 + 2SO4- + KIO3
(A)
New answer posted
9 months agoContributor-Level 10
2. (A) = FeCr2O4
(B) = Na2CrO4
(C) = Na2Cr2O7
(D) = K2Cr2O7
4FeCrO4 + 8NaCO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
(A) (B)
2NaCrO4 + 2H + → Na2Cr2O7 + 2Na + + H2O
Na2Cr2O7 + KCl→ K2Cr2O7 + 2NaCl
(C) &
New answer posted
9 months agoContributor-Level 10
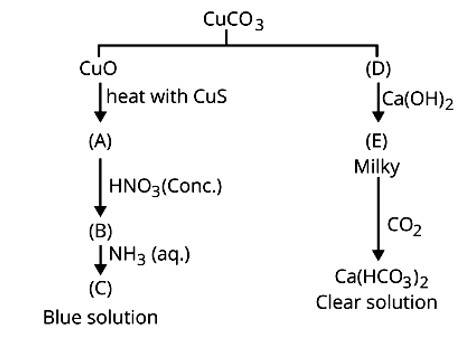
1. CuCO3 ![]() CuO + CO2
CuO + CO2
(D)
Ca (OH)2 + CO2 → CaCO3 + H2O
(E)
CaCO3 + CO2 + H2O → Ca (HCO3)2
clear sol.
CuO + CuS ![]() 3Cu + SO2
3Cu + SO2
(A)
Cu + 4HNO3 → Cu (NO3)2 + 2NO2 + 2H2O
&nb
New answer posted
10 months agoNew answer posted
10 months agoContributor-Level 10
Students need to practice the Class 12 Chemistry previous year question to identify the important questions. Some of important questions are reactions of and in acidic, basic, and neutral medium, lanthanide contraction and its consequences, balancing redox reactions, etc.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers