The p-Block Elements
Get insights from 81 questions on The p-Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The p-Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
The compounds X, A, B, C and D are aluminium, aluminium hydroxide, sodium tetrahydrozoaluminate (III), aluminium chloride and alumina.
Aluminium reacts with NaOH to form white PPT of Al (OH)3→.
2Al + 3NaOH → Al (OH)3→ + 3Na+
Al (OH)3→ reacts with NaOH to form Na+ [Al (OH)4→]→.
Al (OH)3 + NaOH → Na+ (Al (OH)4)→
Al (OH)3→ reacts with HCL to form AlCl3→.
Al (OH)3 + 3HCl → AlCl3 + 3H2O
When Al2→O3→ is heated, Al2→O3→ is obtained.
2Al (OH)3 → Al2O3 + 3H2O
New answer posted
8 months agoContributor-Level 10
Tl belongs to group 13 and shows both the oxidation state +1 and +3 due to inert pair effect. Tl forms basic oxide like group I elements. TlO2 is strongly basic.
New answer posted
8 months agoContributor-Level 10
(a) Neutral — CO
Acidic — B2O3, SiO2, CO2
Basic — Tl2O3
Amphoteric — Al2O3, PbO2
(b)-CO does not react with acid as well as base at room temperature.
Being acidic B2O3, SiO2 and CO2 react with alkalis to form salts.
B2O3 + 2NaOH à 2NaBO2 + H2O
SiO2 + NaOH à 2Na2SiO3 + H2O
CO2 + 2NaOH à Na2CO3 + H2O
Being Amphoteric, Al2O3, PbO2 react with acids and bases.
Al2O3 + 2NaOH à 2NaAlO2 + H2O
Al2O3 + 3H2SO4à (Al2SO4)3+ 3H2O
PbO2 + 2 NaOH à Na2PbO3 + H2O
2PbO2 + 2 H2SO4à 2PbSO4 + 2H2O + O2
Being basic Tl2O3 dissolves in acids
Tl2O3 + 6HCl à 2TlCl3 + 3H2O
New answer posted
8 months agoContributor-Level 10
Due to poor shielding effect of d-electrons in Ga, the electrons in gallium experience great force of attraction by nucleus as compared to Al. That is why Ga has lower atomic radius as compared to Al.
New answer posted
8 months agoContributor-Level 10
Because there is increase in atomic size on moving from carbon to silicon, the screening effect increases. Thus, the force of attraction of nucleus for the valence electron in silicon decreases as compared to carbon. Therefore, the ionization enthalpy decreases from carbon to silicon.
New answer posted
8 months agoContributor-Level 10
(i) Al reacts with conc. HNO3 to form a very thin film of aluminium oxide on its surface which protects it from further reaction. That is why conc. HNO3 can be transported in aluminium container.
2Al (s) + 6HNO3 (conc.) → Al2O3 (s) + 6NO2 (g) + 3H2O (l)
(ii) NaOH reacts with Al to evolve H2 gas. Thus, the pressure of the gas produced can be used for opening or cleaning clogged drains.
2Al (s) + 2NaOH (aq) + 2H2O (l) → 2NaAlO2 (aq) + 3H2 (g)
(iii) Graphite has layered structure which are held by weak van there Waals forces. Thus, graphite cleaves easily between the layers, therefore it is very soft and slippery. That is why
New answer posted
8 months agoContributor-Level 10
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper catalyst at 537 K, methyl substituted chlorosilanes MeSiCl3, Me2→SiCl2→, Me3→SiCl and Me4→Si are formed.
(b) When silicon dioxide is treated with hydrogen fluoride, first SiF4→ is formed and then hydro fluorosilicic acid is obtained.
SiO2 →+ 4HF → SiF4 →+ 2H2→O
SiF4+2HF → H2SiF6
(c) When CO is heated with ZnO, ZnO is reduced to Zn metal.
CO + ZnO →CO2 →+ Zn
(d) When hydrated alumina is treated with aqueous NaOH solution, it dissolves to form sodium meta aluminate.
Al2→O3→.2H2→O+2NaOH→
New answer posted
8 months agoContributor-Level 10
(a) When borax is heated strongly, it loses water and swells into the white mass, which on further heating melts to form a transparent glassy solid called borax glass and borax bead.
Na2B4O710H2O→ Na2B4O7+ 10H2O
Na2B4O7 → 2NaBO2+ B2O3
(b) When boric acid is added to water, it accepts electrons from –OH ion. Boric acid is sparingly soluble in cold water however fairly soluble in hot water.
B (OH)3→ + 2H2→ O→ [B (OH)4→ ]→ + H3→ O+
(c) Al reacts with dilute NaOH to form sodium tetrahydroxoaluminate (III). Hydrogen gas is liberated in the process.
2Al + 2NaOH + 6H2O→ 2Na+ [Al (O
New answer posted
8 months agoContributor-Level 10
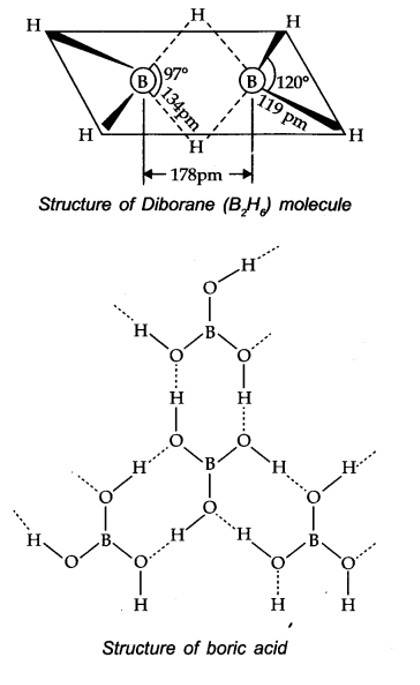
Boric acid contains planar BO33- ions which are linked together through hydrogen bonding shown in the fig.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
