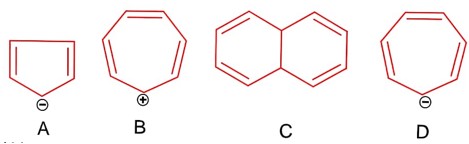
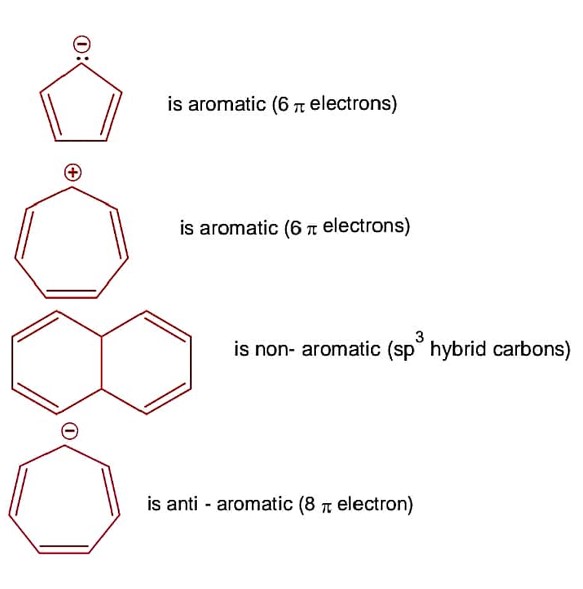
The p-Block Elements
Get insights from 81 questions on The p-Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The p-Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Here, meq of MnO2 = meq of Na2S4O6
Mass of MnO2 in sample = 0.261 g
Percentage of MnO2 in sample =
= 13.05%
New answer posted
6 months agoContributor-Level 10
Pentavalent oxides of group – 15 elements, E2O5 is more acidic than trivalent oxides, E2O3 of the same element.
Acidic strength of trivalent oxides decreases down the group as metallic strength increases.
New answer posted
6 months agoContributor-Level 10
Fluorine forms only one oxoacid which is hypofluorous acid HOF because it shows only – 1 oxidation state. Which is due to its the smallest size among halogens & the highest electronegativity.
New answer posted
6 months agoContributor-Level 10
Structure PCl5 is trigonal bipyramidal,
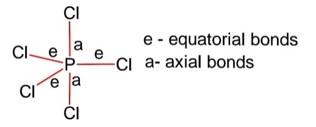
Hybridization of P is sp3d
Equatorial bonds lie in a plane
Axial bonds are longer than equatorial bonds so axial bonds are weaker than equatorial bonds.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers