The p -Block Elements
Get insights from 161 questions on The p -Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The p -Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
- PbCl2 + Cl2→ PbCl4.
This is because Pb can show +2 oxidation state more easily than +4 due to inert pair effect. - PbCl4→ PbCl2 + Cl2
Because Pb2+ is more stable than Pb4+ due to inert pair effect. - PbI4does not exist because I- ion being a powerful reducing agent reduces Pb4+ ion to Pb2+ ion in solution.
Pb4+ + 2I– → Pb2+ + I2
New answer posted
9 months agoContributor-Level 10
Diamond has a crystalline lattice where each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in space and produces a rigid three- dimensional network of carbon atoms. It is very difficult to break extended covalent bonding and, therefore, diamond is a the hardest substance on the earth.
Graphite has layered structure in which the layers are held by van there Waals forces and distance between two layers is 340 pm. Each layer is composed of planar hexagonal rings of carbon atoms. C—C bond length
New question posted
9 months agoNew answer posted
9 months ago11.9. What are electron deficient compounds? Are BCl3 and SiCl4 electron deficient species? Explain.
Contributor-Level 10
Electron deficient compounds are those in which the central atom in their molecule has the tendency to accept one or more electron pairs. They are also known as Lewis acid. BCl3 and SiCl4 both are electron deficient species.
Since, in BCl3, B atom has only six electrons. Therefore, it is an electron deficient compound.
In SiCl4 the central atom Si has 8 electrons but it can expand its covalency beyond 4 due to the presence of d-orbitals.
New answer posted
9 months agoContributor-Level 10
Aluminium reacts with acid as well as base. This shows amphoteric nature of aluminium.
2Al (s) + 6HCl (dil.) →2AlCl3 (aq) + 3H2 (g)
2Al (s) + 2NaOH (aq) + 6H2O (l) →2Na+ [Al (OH)4]– (aq) + 3H2 (g)
New answer posted
9 months agoContributor-Level 10
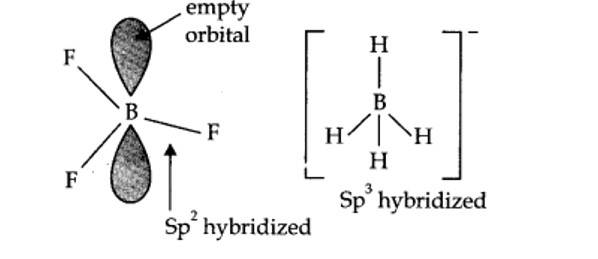
In BF3, boron is sp2 hybridized.
? shape of BF3 = planar.
In [BH4]–, boron is sp3 hybridized, thus the shape is tetrahedral.
New answer posted
9 months agoContributor-Level 10
On heating boric acid above 370 K, it forms metaboric acid, HBO2 which on further heating yields boric oxide B2O3.
H3B2O3 → HBO2 → B2O3
New answer posted
9 months agoContributor-Level 10
Boric acid is a Lewis acid since it accepts electrons from hydroxyl ion of H2O molecule. It is not a protic acid.
B (OH)3 + 2HOH → [B (OH)4]– + H3O+
New answer posted
9 months agoContributor-Level 10
In BCl3, there is only six electrons in the valence shell of B atom. Thus, the octet is incomplete and it can accept a pair of electrons from water and hence BCl3 undergoes hydrolysis. Whereas, in CCl4, C atom has 8 electrons and its octet is complete. That's why it has no tendency to react with water.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
