The p -Block Elements
Get insights from 161 questions on The p -Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The p -Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoNew answer posted
9 months agoContributor-Level 10
(d) CO2 is regarded as anhydride of carbonic acid.
H2CO3 ————> H2O + CO2
New answer posted
9 months agoContributor-Level 10
(d) is an incorrect statement because non-metal oxides are acidic or neutral whereas metal oxides are basic in nature.
New answer posted
9 months agoContributor-Level 10
(c) Boron halides are Lewis acids and can accept a pair of electrons from amines to form addition product.
New answer posted
9 months agoContributor-Level 10
(d) Heavier elementsdo not form pπ– pπ bonds because their atomicorbitals are too large and diffuse to haveeffective overlapping.
New question posted
9 months agoNew answer posted
9 months agoContributor-Level 10
Hydrolysis of aikyltrichlorosilanes gives cross-linked silicones.
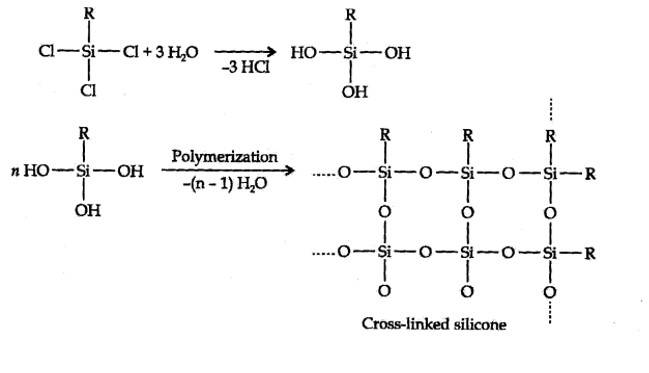
New answer posted
9 months agoContributor-Level 10
Due to inert pair effect, elements of group 14 exhibit oxidation states of +2 and +4. Thus, option (b) is correct.
New answer posted
9 months agoContributor-Level 10
Thermodynamically the most stable form of carbon is graphite, i.e., option (b) is correct.
New answer posted
9 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
