The p -Block Elements
Get insights from 161 questions on The p -Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The p -Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
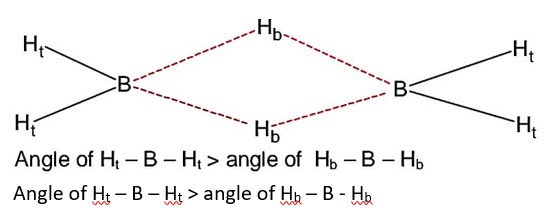
Angle of Ht – B – Ht > angle of Hb – B - Hb
Bond angle
New answer posted
6 months agoContributor-Level 10
For same thermodynamic tendency of reduction
Assume
Comparing x = 2.16 and 2x = 4.32
Nearest integer is 4.
New answer posted
6 months agoContributor-Level 10
Bond dissociation enthalpy is the energy required to break 1 mole of bond according to experimental data
Bond energy : Cl2 > Br2 > F2 > l2
New answer posted
6 months agoContributor-Level 10
B2H6 has 4 2 c -2e bonds and 2 3c-2e bonds.
Bridging (B-H) bonds have more value of bond- length then terminal (B – H) bonds
Bridging bonds are in one plane, but terminal bonds are in perpendicular plane.
Due to presence of (3c-2e) bonds, it behaves as electrons deficient and prone to get attached by lewis base.
New answer posted
6 months agoContributor-Level 10
The highest industrial consumption of hydrogen gas is in the synthesis of ammonia gas (Having manufacturing of N-based fertizers)
New answer posted
6 months agoContributor-Level 10
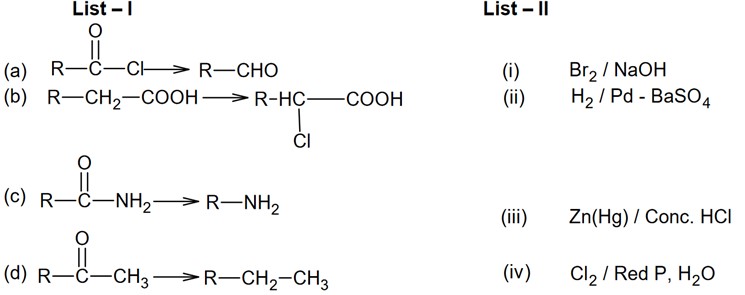
Acidic oxide -> Cl2O7
Neutral oxide -> N2O, NO
Basic oxide ->Na2O
Amphoteric oxide -> As2O3
New question posted
6 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers