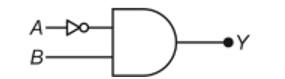
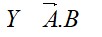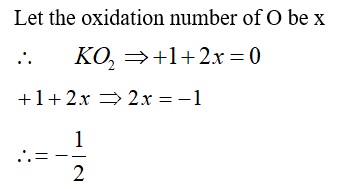The p -Block Elements
Get insights from 161 questions on The p -Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The p -Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Proteins on hydrolysis gives α-amino acid because α-amino acids are building block of proteins. It is also fact that amino acids contain both –NH2 and –COOH group.
New answer posted
4 months agoContributor-Level 10
Amphoteric oxides are those which can react with both acid and base
SnO2, PbO2 and Al2O3 are amphoteric oxide
SiO2, P2O5, CO2 are acidic oxides
CO, NO and N2O are neutral oxides
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers