Thermodynamics
Get insights from 325 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
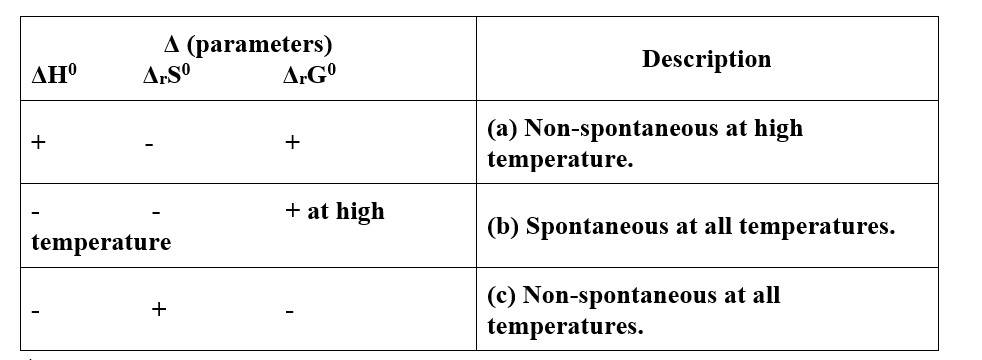
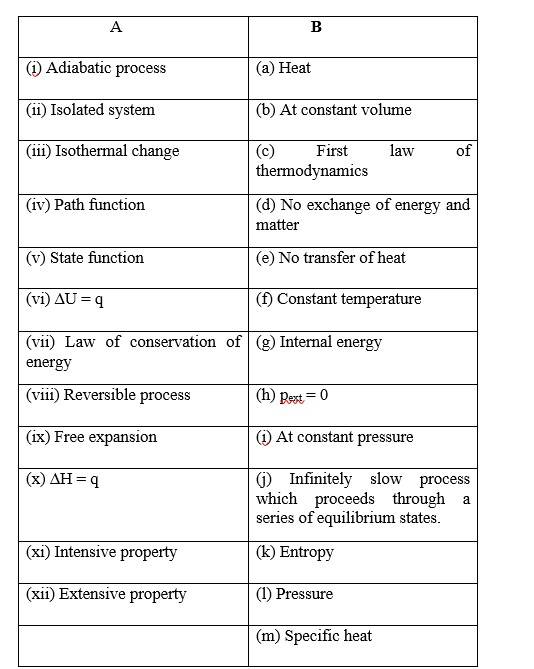
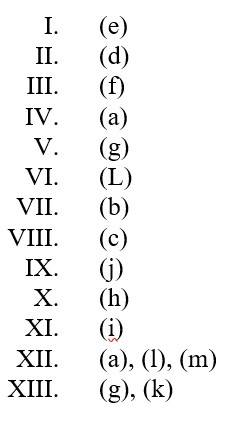
(i)- (c)
(ii)- (a)
(iii)- (b)
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
(i) (b)
(ii) (c)
(iii) (a)
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (i) and (iii)
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iv)
For
isothermal reversible change
q= -w = nRTln =2.303nRTlog
= = =2
For
isothermal expansion of ideal gases, ? U = 0
Since,
temperature is constant this means there is no change in internal energy.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iii) and (iv)
Gas expands to fill the available space spontaneously, and burning of carbon to carbon dioxide is spontaneous
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (i) and (ii)
In an exothermic reaction, heat is evolved, and system loses heat to the surroundings. Therefore, qp will be negative and? rH will also be negative. Hence, option (i) and (ii) is the correct answer.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (i)
The laws of thermodynamics deal with energy changes of macroscopic systems involving a large number of molecules rather than microscopic systems containing a few molecules. Laws of thermodynamics apply only when a system is in equilibrium or moves from one equilibrium state to another equilibrium state.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (ii)
positive for a spontaneous reaction
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (i)
Enthalpy of sublimation can be shown as
Solid | Liquid | Vapour
Hence, Enthalpy of sublimation of a substance is equal to enthalpy of fusion + enthalpy of vaporisation.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers