Thermodynamics
Get insights from 325 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
0.25 = 1 -
0.25 = 1 -
T1 = 400 k
Now, efficiency increases by 100%
= 0.25 * 2
= 0.50
0.50 = 1 -
(600 – 400) = 200 k or 200° C
New answer posted
7 months agoContributor-Level 10
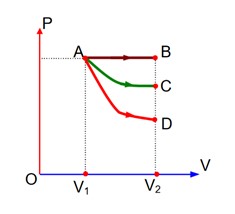
Process-AB Isobaric,
Process-AC Isothermal, and
Process-AD? Adiabatic
W2 < W1 < W3
New answer posted
7 months agoContributor-Level 10
Water has only two lone pair and XeF4 has two lone pair electron in opposite plane of the central atom.
New answer posted
7 months agoContributor-Level 10
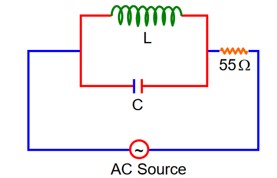
At Resonance
XL = XC
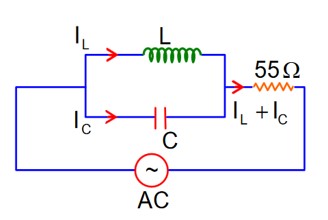
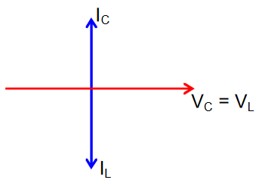
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
Therefore current through R circuit at resonance will be zero
New answer posted
7 months agoContributor-Level 10
85gm NH3 = 5 moles of NH3
Enthalpy change for 1 mol = 23.4 kJ
Then enthalpy change for 5 mol = 23.4 * 5 = 117 kJ
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers