Thermodynamics
Get insights from 325 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
The presence of reactants in a closed vessel made of conducting material e.g., copper or steel is an example of a closed system.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Thermodynamics is not concerned about how and at what rate these energy transformations are carried out but is based on initial and final states of a system undergoing the change. Laws of thermodynamics apply only when a system is in equilibrium or moves from one equilibrium state to another equilibrium state.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
We know that the amount of work done =-pext? V
On substituting the values in the formula, we get,
-2bar* (50-10)L=-80Lbar
According to the described problem,1 LBar = 100J
Therefore, -80 L bar= (-80*100)= -8000J
= -8kJ, which is the amount of work done
The significance of the negative sign states that the work is done on the surroundings of the system. In the case of reversible expansion, the work done will be more.
New answer posted
8 months agoContributor-Level 10
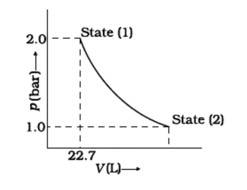
This is a Short Answer Type Questions as classified in NCERT Exemplar
We can conclude from the figure that this change is a reversible change.
Now,
W= -2.303nRT log
But, p1V1 = p2V2 = = = =2
W= -2.303nRT log
= -2.303 *8.314*1*298*log2
= -2.303 *8.314*298*0.3010J
= -1717.46J
New answer posted
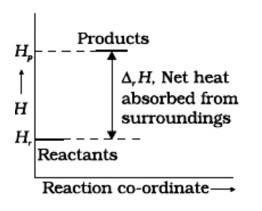
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
No, for the state of spontaneity, the enthalpy change is not the only criteria. Entropy also needs to be taken into account here.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Throwing a stone from ground to roof
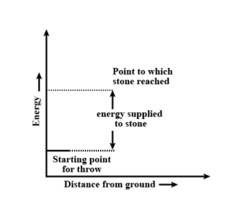
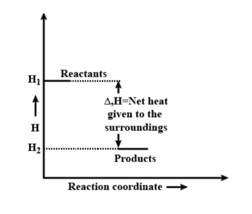
b) the reaction involved is a process where the energy decreases after the reaction. It can be represented as:In process b), potential energy/enthalpy change is a contributing factor to the spontaneity.
New answer posted
8 months agoContributor-Level 10
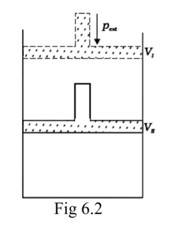
This is a Short Answer Type Questions as classified in NCERT Exemplar
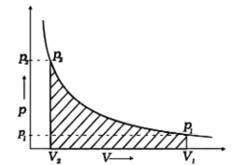
When a process can be reversed by bringing an extremely small change in it, we call it a reversible process. The pressure-volume graph can be used to calculate the work done. The pressure is not constant, and changes in infinitesimal amounts as compression happens from initial volume Vi to the final volume Vf. The below graph depicts the work done with the shaded area.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
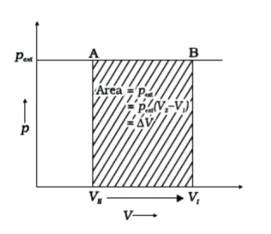
Assumption: Cylinder is filled with one mole gas, and the piston is frictionless. Let the pressure of gas inside be p and the volume of gas be V_ {I}.
Piston is moved towards the inside to make the external pressure (P_ {ext}) equal to p. Now, let us assume that this change takes place in a single step, hence, V is the final volume. The work done by the piston is depicted in the graph shown below by shading the area.
PextΔV= AV1 (V1-V2)
New answer posted
8 months agoContributor-Level 10
Chemistry NCERT Exemplar Solutions Class 11th Chapter six
Standard molar enthalpy of formation is the enthalpy change for the formation of one mole of a compound from its most stable states or reference states. As per the given information in the question, the standard enthalpy for the given equation is – 572 kJ mol–1
Now the enthalpy of formation for H2O will be half the enthalpy of the value in the given equation. So now we can calculate that
? fH? = = -286KJ/mol
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
As per the information provided in the question, for one mole of CCl4 (154 g), the heat of vaporisation required is 30.5 kJ/mol .
Hence for the vaporisation of 284 g of CCl4, we require:
= 56.2 kJ
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers