Thermodynamics
Get insights from 325 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
Number of moles, n = 5 mol
Temperature, T= 300 K
Initial volume, V1 = 10L
Final volume, V2 = 20 L
Using;
Work done; w = -2.303 nRT log10
= -8630 J
So, magnitude of work done is 8630 J.
New answer posted
8 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
option (i)
Explanation: The entropy of a liquid reduces as it crystallises. Because the molecules are more organised in crystalline form.
New answer posted
8 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (ii)
Explanation: The energy factor for a spontaneous process should be favourable (i.e., -ve) and the randomness should be positive.
New answer posted
8 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
option (ii)
Explanation: The enthalpy of the reactants is always greater than the enthalpy of the product in a combustion reaction.
New answer posted
8 months agoContributor-Level 10
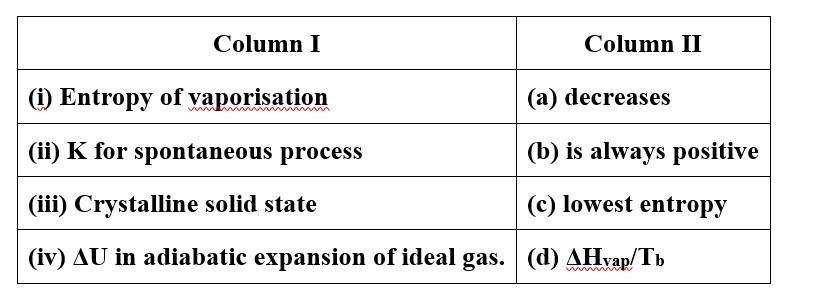
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (b), (d)
(ii)- (b)
(iii)- (c)
(iv)- (a)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers