Thermodynamics
Get insights from 325 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
Let x gm is burnt
Moles = x/280
Heat released by x/280 mol = 2.5 * 0.45 kJ
Heat released by 1 mol =
x = 35 gm
New answer posted
7 months agoContributor-Level 10
2O3(g) -> 3O2
t = 0 a moles 0
-0.5a mole +0.75a mole
At Eq. 0.5a mole 0.75a mole
Total moles at eq = 0.5a + 0.75 a = 1.25a
New answer posted
7 months agoContributor-Level 10
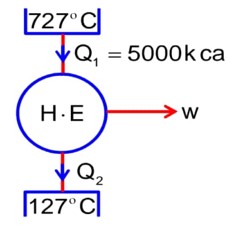
T1 = 727 + 273 = 1000k
T2 = 127° + 273 = 400 k
Q1 = 5 * 103 k cal
w = 12.6 * 106 J
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers