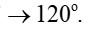Chemistry Chemical Bonding and Molecular Structure
Get insights from 133 questions on Chemistry Chemical Bonding and Molecular Structure, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Chemical Bonding and Molecular Structure
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Bond angles give some idea regarding the distribution of orbitals around the central atom in a molecule/complex ion and hence it helps us in determining its shape
New answer posted
3 months agoContributor-Level 10
PF5, PCl5, PBr5, Fe (CO)5 Þ Trigonal bipyramidal
BrF5 Þ Square pyramidal
[PtCl4]2– Þ Square planar
SF6 Þ Octahedral
New answer posted
3 months agoContributor-Level 10
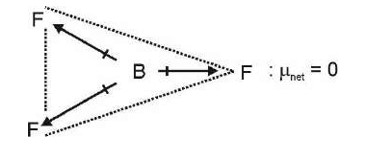
Polarisation power µ for K+, polarising power is least and ionic character is maximum.
New answer posted
3 months agoContributor-Level 10
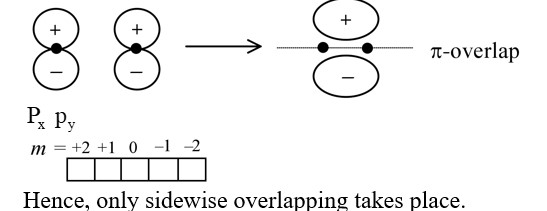
For overlap, the lobes of the atomic orbitals are perpendicular to the line joining the nuclei.
New answer posted
3 months agoContributor-Level 10
Kindly go through the solution
B2 ->s1s2 s*1s2 s2s2 s*2s2 p2p1= p2p1
New answer posted
3 months agoNew answer posted
4 months agoContributor-Level 10
NH+4does not have vacant orbitals, therefore it cannot behave as electrophile.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers