In Chemistry, we have noticed how substances change during a chemical reaction. But have you ever thought of how fast or slow these changes occur? This is the rate of chemical reaction, which tells how fast reactants are consumed and products are formed in a chemical change.
The rate of reaction depends on various factors such as temperature, the presence of a catalyst, and the concentration of reactants. Aslo, these factors affect the collision number and energy between particles; this concept is explained by the collision theory. Knowledge of the rate of reaction helps in controlling the reaction in real life.
Important Links:
| NCERT Class 11 notes | |
| Chemistry Class 11 NCERT notes |
Keypoints:
- Fast reaction: Takes place over is short duration
- Slow reaction: Required a long time to complete.
Rate of Reaction formula:
Rate = Change in concentration / time taken
- Rate of Reaction: Definition and Significance
- Unit of Rate of Reaction
- Types of Reaction Rates
- Rate Expression for Specific Reactions
- Instantaneous rate of reaction
- Pseudo First-Order reaction
- Factors Affecting Rate of Reaction
- Practice Questions
- Summary Table
Rate of Reaction: Definition and Significance
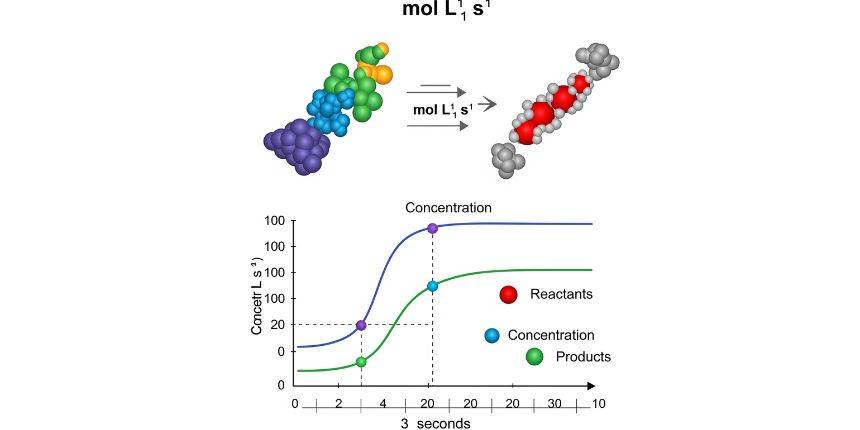
In a chemical process, the speed at which a reactant converts into a product is known as the rate of reaction. The rate of reaction is calculated by dividing the change in concentration of a substance by a unit of time.
Aslo read: NCERT Solutions | Class 11 Chemistry NCERT Solutions
Rate of reaction = Change in concentration / time taken
The rate of reaction is measured in mol (or atm for gases, using ).
Significance of Rate of Reaction
The importance of the rate of reactions is
- If we know the rate of reaction, then it is possible to control the speed of the chemical reaction as per our needs.
- In real life, the best example of the rate of reaction is cooking food. When the heat is high, food starts cooking fast and similarly, it is reverse when the heat is low. We know that in high heat, food can get burned so we can prevent this by controlling the heat.
- In industries, we can reduce the waste by controlling the rate of reaction and increasing the production rate.
Unit of Rate of Reaction
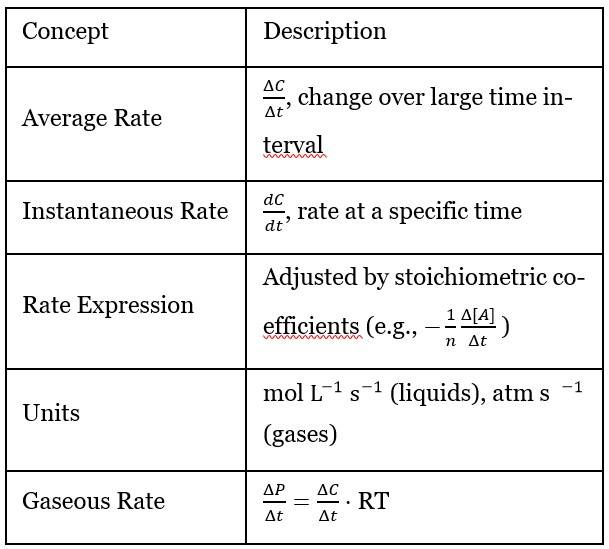
The unit of rate of reaction depends on how it is measured. Usually, the rate is defined as the change in concentration of a reactant or product per unit time. Concentration is typically measured in moles per litre (mol/L) and time in seconds (s). The standard unit of rate of reaction is mol·L⁻¹·s⁻¹ (moles per litre per second). For a gaseous reaction unit of rate of reaction is expressed in atm/s.
Types of Reaction Rates
The rate of reaction is classified in various ways. Below are the types of rate of reaction.
Average Rate of Reaction:
- The rate is defined as the change in concentration of reactants or products over a time interval :
- For a reaction , the rate is:
- Average rate of reaction Units:
or
(Page 1).
Example: For , if changes from 0.03 M to 0.02 M in 25 min (Page :
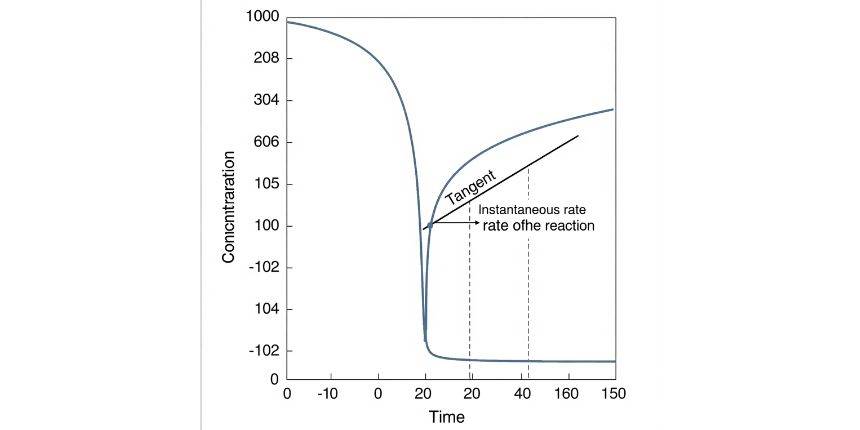
Instantaneous Rate of Reaction:
- Rate is defined at a specific moment in time
- Rate changes throughout the reaction
- Calculated as the limit of the average rate over a very short interval ):
- For :
Initial Rate of Reaction:
- Rate of reaction at the initial at the beginning of the reaction, i.e. t=0"
- The initial rate helps in determining the reaction order and rate constant.
Rate Expression for Specific Reactions
For a reaction like , the rate is:
For , if the rate of decomposition of N2O5 is , then:
Instantaneous rate of reaction
The rate of reaction at a specific moment in time during a chemical reaction is referred to as the instantaneous rate of reaction. It shows how fast a reactant is consumed or a product is formed at a particular point. We can determine the instantaneous rate of reaction by finding the slope of the tangent to the concentration vs time graph at an exact point. It is useful in reactions where the rate changes quickly. Instantaneous rate of reaction is mol·L⁻¹·s⁻¹.
Pseudo First-Order reaction
A chemical reaction which is a higher-order reaction, but the concentration of molecules hardly changes because of the large number, so it looks like a first-order reaction.
In technical terms, x+y is not equal to 1 (one).
For example: 2+2=4
Factors Affecting Rate of Reaction
The factors affecting the rate of reaction are:
- Concentration: In a chemical reaction, when the concentration of reactants is increased, the reaction rate also increases.
- Pressure: For gases, when the pressure is increased, the concentration of gas molecules increases and there is a rise in reaction rate.
- Temperature: When the temperature is increased by every 10°C, the rate of reaction doubles or triples.
- Surface Area: If the surface area of the solid reactant is increased, the rate of reaction also increases.
Practice Questions
Summary Table
Chemistry Chemical Kinetics Exam