Chemistry Coordination Compounds
Get insights from 136 questions on Chemistry Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Statement – I True
Statement – II False
Dimethyl glyoxime is the bidentate anionic ligand.
New answer posted
5 months agoNew answer posted
5 months agoContributor-Level 10
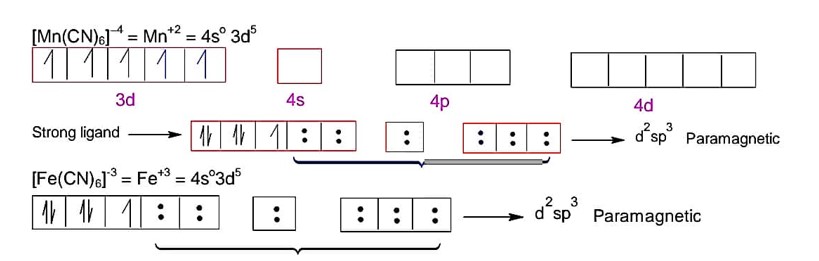
NH3 behaves as strong field ligand with Co2+ & Co3+
It has one unpaired electorn.
It has no unpaired electrons
So, total unpaired electrons in both compounds is 1.
New answer posted
5 months agoContributor-Level 10
(A) - Paramagnetic & coloured
- Diamagnetic & colourless
- Diamagnetic & colourless
(B) - Diamagnetic & colourless
- Diamagnetic & colourless
- paramagnetic & coloured
(C)
All are diamagnetic & colourless
(D)
All are paramagnetic & coloured
All d- & f block paramagnetic cations are coloured also.
New answer posted
5 months agoContributor-Level 10
For Ni2+, crystal field CFSE magnitude shows the magnitude of absorbed light. Following are the energy absorbed order
Hence order of colour of compounds are.
New answer posted
5 months agoContributor-Level 10
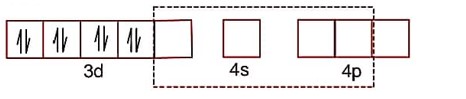
dsp2 hybridisation
Paring developed due to strong ligand effect.
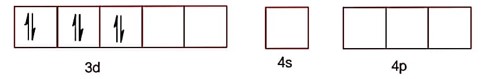
NiCl2.6H2O
Ni2+ ->3d84s0
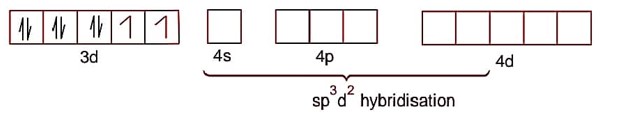
Ni2+ -> 3d84s0
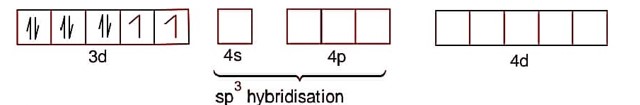
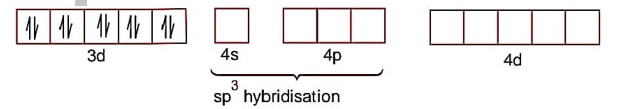
[Ni (CO)4]
Ni->3d84s2
New answer posted
5 months agoContributor-Level 10
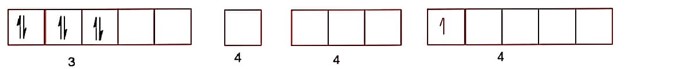
In strong field of octahedral complex of Fe2+, the electronic configuration is,
Number of unpaired electrons are zero in the presence of strong field ligand.
Hence, spin only magnetic moment =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers