Chemistry Coordination Compounds
Get insights from 136 questions on Chemistry Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
x + 4 * 0 – 2 * 1 = +1
x = +3
Fe+3 = 4s0 3d5
In presence of strong ligand, i.e, CO.
Spin only magnetic moment
New answer posted
5 months agoContributor-Level 9
Co has +2 oxidation state
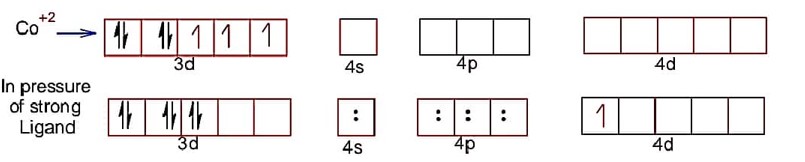
Hence, electronic configuration of CO2+ is . In complex given ligand CN is strong hence, after pairing in d-subshell, total number of unpaired electron =
spin magnetic moment =
New answer posted
5 months agoContributor-Level 10
Calamine is the ore of zinc i.e. ZnCO3.
Malachite is the ore of copper i.e. CuCO3.Cu (OH)2.
New answer posted
5 months agoContributor-Level 9
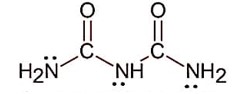
Biuret is
Denticity is 2 of terminal -NH2 only, because middle –NH will undergo in cross conjugation.
New answer posted
5 months agoContributor-Level 10
t = 00.8 M
0 5 * 10-8 M 0.8M
Concentration of NH3 added =
Volume of solution = 2L
Moles of NH3 added = 2 * 2 mol = 4 mol.
New answer posted
5 months agoContributor-Level 10
MCl3.2L => octahedral complex
It means one Cl- ion present in ionization sphere.
For octahedral complex co-ordination numbers is 6
is bidentate ligand i.e. its denticity is 2.
New answer posted
5 months agoContributor-Level 10
Stability constant of complex =
the nearest value of dissociation constant = 5 * 10-14
Ans. = 5
New answer posted
5 months agoContributor-Level 10
Violet colour
Blood red colour
Prussian blue colour
Yellow colour
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers