Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
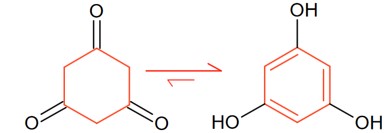
Aromaticity drives the highest enolic percentage of given structure:
New answer posted
5 months agoContributor-Level 10
P4 + 3NaOH + 3H2O
Oxoacid is H3PO2 it is also known as hypophosphorous acid or phosphinic acid.
New answer posted
5 months agoContributor-Level 10
Due to common ion effect solubility of AgCl will decreases in KCl, AgCl and AgNO3 but in deionized water, no common ion effect will takes place so maximum solubility.
New answer posted
5 months agoContributor-Level 10
(a) Sucrose → α-D glucose and β-D fructose
(b) Lactose → β-D- galactose and β-D glucose
(c) maltose → α-D glucose and α-D-glucose
New answer posted
5 months agoContributor-Level 10
It does not contains 2nd most abundant element by weight in earth crust because that is Si Calgon
New answer posted
5 months agoContributor-Level 10
Ceric ammonium nitrate is used to test alcohol while CHCl3/alc. KOH is used to test 1° amine
New answer posted
5 months agoContributor-Level 10
Due to common ion effect solubility of AgCl will decreases in KCl, AgCl and AgNO3 but in deionized water, no common ion effect will takes place so maximum solubility.
New answer posted
5 months agoContributor-Level 10
Phenolphtalein is a pH dependant indicator. It is a weak acid which is colourless in acidic medium but gives pink colour in basic medium. The pink colour is due to its conjugate form. Phenolphthalein dissociates in basic medium. Therefore, assertion is true but reason is false.
New answer posted
5 months agoContributor-Level 10
At equilibrium rate of forward and backward reaction becomes equal.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers