Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Isotopes are the atom of same element with different atomic mass.
New answer posted
5 months agoContributor-Level 10
In extraction of copper from copper pyrite (CuFeS2).
FeS converted to FeO as;
Then FeO is removed as slag by given reaction
FeO + SiO2 ®
New answer posted
5 months agoContributor-Level 10
· Cl2O7 being non-metallic oxide is acidic in nature.
· Na2O being metallic oxide is basic in nature.
· Al2O3 is an amphoteric oxide
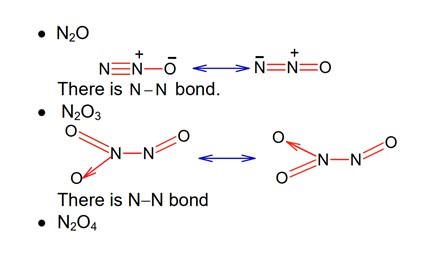
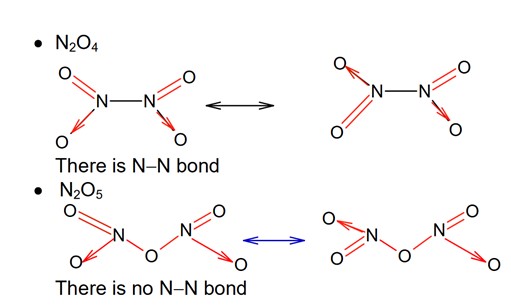
· N2O is neutral oxide
New answer posted
5 months agoContributor-Level 10
Metallic suphides i.e CdS are negatively charged colloidal solution.
Starch colloid is macro molecular colloidal solution.
Fe2O3. xH2O sol is positively charged colloidal solution.
Cheese is an example of gel.
New answer posted
5 months agoContributor-Level 10
Wt of liquid = 135 – 40 = 95 gm
Volume of liquid =
Hence volume of vessel = 100 ml = 0.1 lit from ideal gas equation,
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers