Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
In the electrolysis of sulphuric acid, the following reactions occur
2SO42− (aq)→S2O82− (aq) + 2e− E? Cell =1.96V
2H2O (l)→O2 (g)+4H+ (aq) + 4e− E? Cell =1.23V
The reaction will lower the value of E? Cell is preferred at anode so the second reaction is feasible.
H+ + e− → H2 E? Cell = 0.00V
At the cathode, reduction of water occurs. Therefore, in dilute sulphuric acid solution, hydrogen will be reduced at the cathode.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (ii, iv)
The lesser the E? value of the redox couple higher is the reducing power
For Cu2+ + 2e−→Cu; E? =0.34V
For 2H+ + 2e−→H2 E? =0.00V
Since the second redox couple has less standard reduction potential than the first so it can be concluded that the redox couple is a stronger oxidizing agent than H+/H2 and copper cannot displace H2 from acid.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iv)
The electrolysis of aqueous sodium chloride is as follows
NaCl→Na+ + Cl−
H2O→H+ + OH−
At the cathode, the reaction goes this way,
H2O + e− → H2 + OH−
At the anode, the reaction goes this way,
Cl− (aq)→ Cl2 (g)+ e− E? Cell =1.36V
2H2O (g)→O2 (g) + 4H+ (aq) + 4e− E? Cell =1.23V
At the anode, the reaction with a lower E? value will be preferred and oxidation of oxygen is a slow process and requires high voltage so the first reaction will take place.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (ii)
Given: Salt ammonium hydroxide
Apply Kohlrausch Law
Kohlrausch law of states that limiting molar conductivity of any salt species is equal to the sum of the limiting molar conductivity of cations and anions of the electrolyte
Λ0m (NaOH) = Λ0m (NH4+) + Λ0m (OH-) + Λ0m (Na+) + Λ0m (OH-) - Λ0m (Na+)- Λ0m (Cl-)
Λ0m (NH4OH) = Λ0m (NH4OH) + Λ0m (NaOH) – Λ0 (NaCl)
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (i)
In the lead storage battery, a reverse reaction occurs and lead sulfate is formed at the anode, and at the cathode, it is converted into lead and lead oxide respectively. The reaction is as follows
At the cathode, the reaction goes this way,
PbSO4 (s)+2e−→Pb (s)+SO42− (aq), Reduction process
At the anode, the reaction goes this way,
PbSO4 (s)+2H2O→PbO2 (s)+SO42−+2H+ +2e, oxidation process
The overall reaction is as follows;
2PbSO4 (s)+2H2O→Pb (s)+PbO2 (s)+4H+ (aq)+2SO42− (aq)
Therefore, at anode lead sulfate is reduced to lead.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iv)
The cell constant is described as the ratio of the length of the object and the area of cross-section. It can be written as follow
G=
As l and A remains constant for an object it can be inferred that the cell constant for a conductivity cell remains constant for a cell.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct Option: (iii)
Ans: Given: One mole of aluminium
Calculate the number of electrons required to convert Al2O3 to Al
It is the charge required to obtain one mole of aluminium from Al2O3
The dissociation of aluminium oxide is as follows;
Al2O3→2Al3++3O2−
Al3++3e−→Al
Therefore 3Fcharge is required to obtain one mole of aluminum from Al2O3.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (i)
According to the given table, ![]() has the most negative value among the given species. So
has the most negative value among the given species. So
Cr3+ is the most stable oxidized species.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iv)
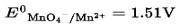
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
According to the electrochemical series, the lower the reduction potential higher is the reducing power.
Therefore, the order of reducing power is Mn2+ < Cl < Cr3+ < Cr
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
