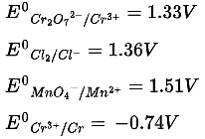Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
According to the electrochemical series higher the positive value of standard reduction potential of metal ion, the higher is the oxidizing power
Therefore, MnO4− is the strongest oxidizing agent.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (ii)
According to the electrochemical series and standard reduction potential of metal, the higher the negative value of standard reduction potential stronger will be the reducing agent
In the given options, the standard reduction potential of chromium has the highest value so it is the strongest reducing agent.
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i) Conductivity of solution depends upon the size of ions as more is the size of ion, mobility decreases, and conductivity decreases so this option is incorrect.
(ii) Viscosity is the measure of the resistance of a liquid to flow. Greater will be the viscosity of solvent, less will be the flow of electrons and lesser will be the conductivity. Therefore, this option is incorrect.
(iii) Conductivity depends on the solvation of ions in the solution. Greater is the solvation, lesser will be the conductivity of the solution. Therefore, this option
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
On the application of external potential, an increased reaction starts till the opposing voltage becomes 1.1V.
However, further increase in the external potential leads the reaction to start in an opposite direction functioning as an electrolytic cell which is a device that uses electrical energy for carrying out electrochemical reactions.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iv)
(i) Inert electrodes like graphite are a source of electrons in a reaction. It doesn't participate in a chemical reaction. Therefore, this option is incorrect
(ii) Inert electrode chemically doesn't participate in the reaction but it provides a surface either for oxidation and reduction reaction. Therefore, this option is incorrect
(iii) Inert electrodes provide surface for conduction of electrons so this option is incorrect.
(iv) Inert electrodes cease transfer of electrons for oxidation or reduction but not for redox reaction. Therefore
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
The difference between electrode potential potentials of two electrodes when no current is drawn through the cell is termed as cell emf. It is the energy provided by a cell per coulomb of charge passing through it. It is the maximum potential difference between the electrodes of a cell.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i) The properties which don't depend on the mass of species are termed intensive properties. ECell is an intensive property that doesn't depend on the mass of the species.? rG is an extensive property as it depends on the mass of the species. Therefore, this option is incorrect
(ii) ECell is an intensive property but? rG is an extensive property so this option is incorrect
(iii) The properties which don't depend on the mass of species are termed intensive properties ECell is an intensive property that doesn't depend on the mass of the species
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
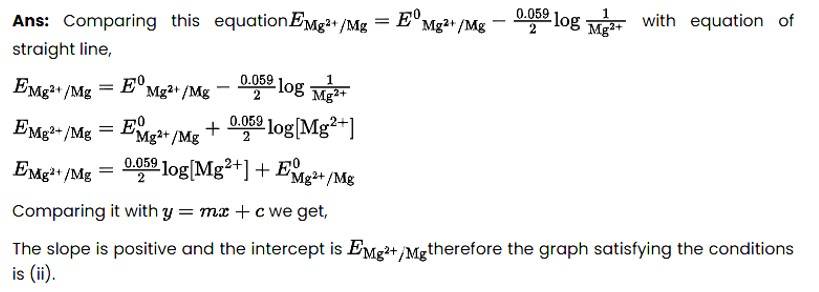
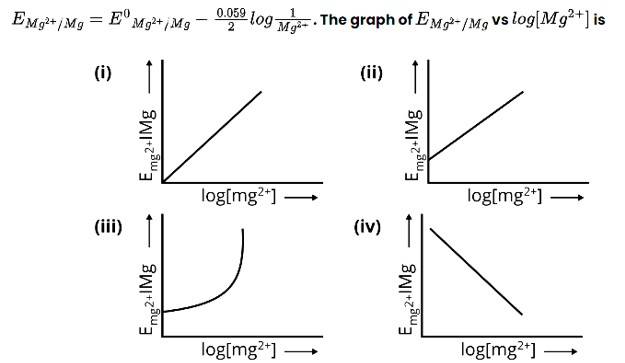
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i) For measurement of electrode potential standard conditions must be met which is 1 bar pressure and 1M concentration which is not here so this option is incorrect.
(ii) For measurement of electrodes potential standard conditions must be met which is 1 bar pressure and 1M concentration which is not here so this option is incorrect.
(iii) When copper electrode is connected to SHE it acts as cathode and its standard electrode potential can be measured as follows
E E R- E L
For calculationn of standard potential of a given cell it shoul
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers