Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
n-factor of KMnO4 in acidic medium = 5
n-factor of Mohr's salt = 1
meq of KMnO4 = meq of Mohr's salt
0.01 * 5 * V = 0.05 * 1 * 20
Volume of KMnO4 used, V = 20 mL
So; Volume of KMnO4 left in burette = 50 -20mL
= 30 mL
New answer posted
5 months agoContributor-Level 10
Moles of C = moles of CO2
=
Mass of C =
= 0.261g
Moles of H = 2 *
Mass of H =
Total mass of compound = 0.492g (given)
So; mass of O = (0.492 – 0.216 – 0.049) g
= 0.227g
% of O =
= 46.14%
the nearest integer = 46
New answer posted
5 months agoContributor-Level 10
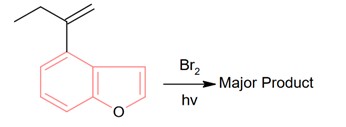
Bromination through free radical mechanism occurs at allylic carbon.
New answer posted
5 months agoContributor-Level 10
(a) will show cis- trans isomerism as;
(b) will not show cis- trans isomerism.
(c) will not show cis- trans isomerism.
(d) will not show cis-trans isomerism.
New answer posted
5 months agoContributor-Level 10
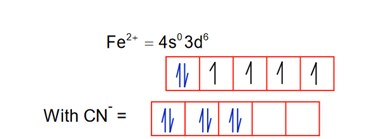
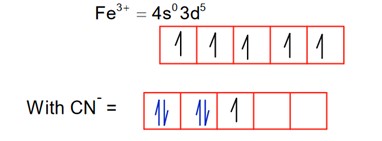
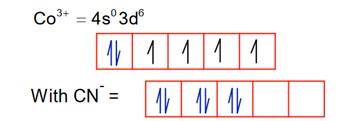
CN is a strong field ligand, so pairing occurs.
1.
So; it is diamagnetic
2.
So; it is paramagnetic.
3.
Ti3+ = 4s03d1
It is paramagnetic
4.
It is diamagnetic
5.
Hence, are paramagnetic.
New answer posted
5 months agoContributor-Level 10
Molar mass of protein, M = 24751 g/mol
Molar mass of glycine = 75 g/mol
So; number of glycine units =
New answer posted
5 months agoContributor-Level 10
For combustion of Mg:
Mg (s) +
Here,
Now using
-601.7 =
So; magnitude of is 600 kJ (the nearest integer).
New answer posted
5 months agoContributor-Level 10
Using : PV = nRT
1.5 * 416 = n * 0.083 * 300
n = 25mol =
25 =
So, molar mass, M = 4 g/mol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers