Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Three, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Three
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Ala – Gly – Leu-Ala- Val
Two amino acid units are connected by peptide linkage. So number of peptide linkage = 4.
New answer posted
5 months agoContributor-Level 10
Number of moles of gas =
Mass of nitrogen =
% of N in organic compound =
New answer posted
5 months agoContributor-Level 10
In acidic solution
Difference in oxidation state of Mn is product
= 7 – 4 = 3
New answer posted
5 months agoContributor-Level 10
Baryte – BaSO4 [Sulphate based ore]
Galena – PbS
Zinc blende – ZnS
Copper pyrite – CuFeS2
New answer posted
5 months agoContributor-Level 10
Number of moles of CH3COOH Adsorbed on 0.6 gm wood charcoal.
= 20 * 10-3 moles
Mass of CH3COOH absorbed = 20 * 10-3 * 60 = 1.2 gm
Mass of CH3COOH adsorbed per gram =
New answer posted
5 months agoContributor-Level 10
Eq.(5 – x) mole x molex mole
Total number of moles at equilibrium
= 1107 * 103 atm
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers

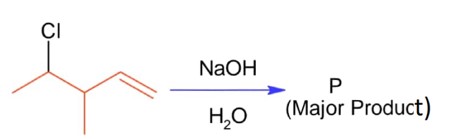
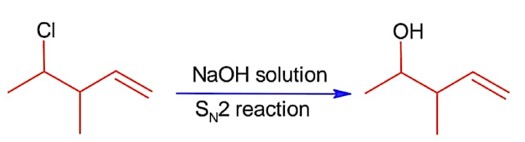 ffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffff
ffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffffff