Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
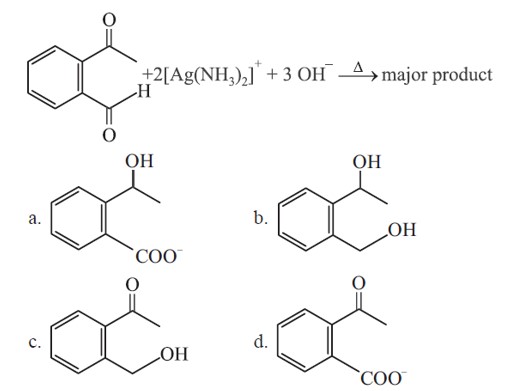
Tollen's reagent oxidises aldehydes into carboxylate ion whereas ketone is not oxidised by tollen's reagent.
New answer posted
3 months agoContributor-Level 10
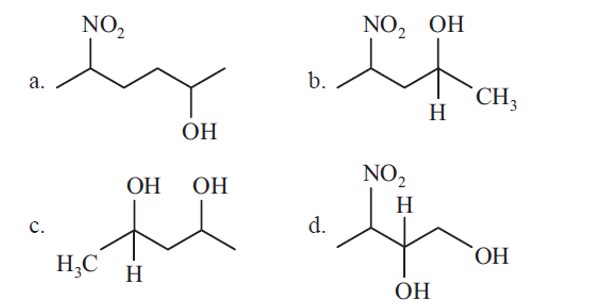
Formation of Conjugated diene in option 3 make the given reactant most reactive towards dehydration in acidic conditions.
New answer posted
3 months agoContributor-Level 10
Ethylene diamine, en is bidentate, chelating ligand. Chelating ligands increase stability due to higher entropy factor.
New answer posted
3 months agoContributor-Level 10
The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc? O? to Mn? O? CrO is basic but Cr? O? is amphoteric.
Note: All the transition metals except scandium form MO oxides which are ionic (Only for 3d series, this statement is true)
Hence, (A), (C) and (D) are incorrect. But not given in the options.
New answer posted
3 months agoContributor-Level 10
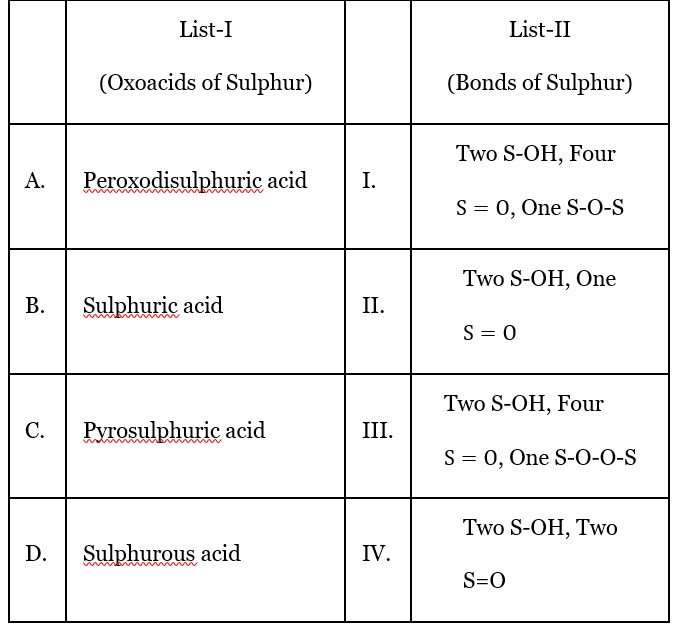
H? S? O? , peroxodisulphuric acid (Two S-OH, Four S = O, One S – O – O – S )
H? SO? , sulphurous acid (Two S-OH, One S = O bond)
H? S? O? , pyrosulphuric acid (Two S-OH, Four S=O, One S-O-S bond)
H? SO? , sulphuric acid (Two S – OH, Two S = O )
New answer posted
3 months agoContributor-Level 10
Fe? O? + CO → 2FeO + CO?
The above reaction takes place at 500 – 800 K in blast furnace.
New answer posted
3 months agoContributor-Level 10
Pumice stone is an example of solid sol. In this type of colloid, the dispersion medium is solid and the dispersion phase is gas.
New answer posted
3 months agoContributor-Level 10
One edge of an cube is common to four unit cells. Hence One edge centre OV contribute 1/4 to one unit cell.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers