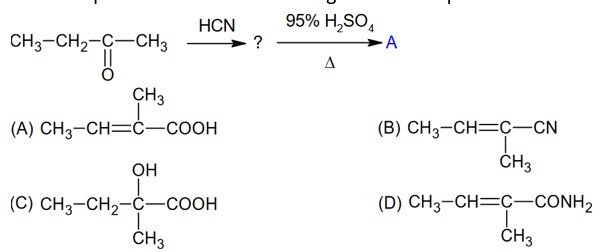
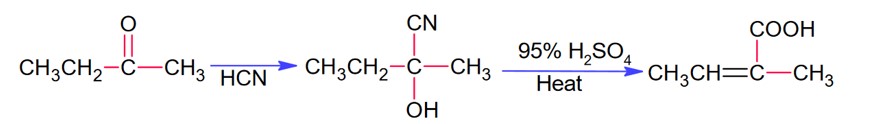
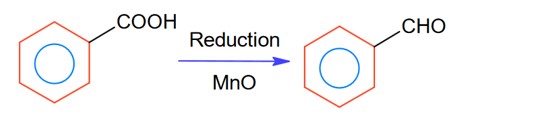
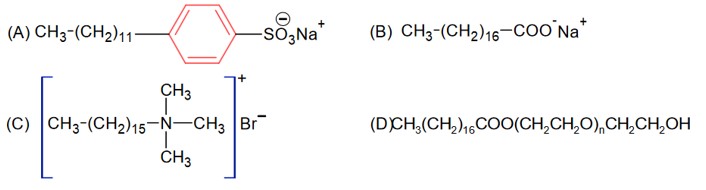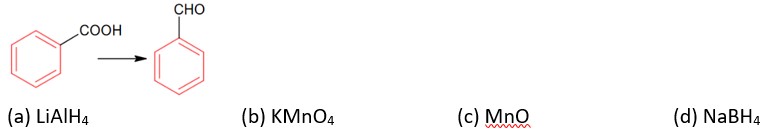Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
When alpha particles pass through the gold foil, alpha particle deflected because according to Thompson model positive charge dispersed through out the atom.
New answer posted
3 months agoContributor-Level 10
Characteristics of crystalline solids:-
Crystalline solid have long range order.
Crystalline solid are anisotropic in nature.
Crystalline solid have definite heat of fusion.
Characteristics of amorphous solids:-
Amorphous solids have short range of order.
Amorphous solids are sometimes called pseudo solids.
Amorphous solids soften over a range of temperature.
New answer posted
3 months agoContributor-Level 9
CH3 – (CH2)16 – COONa is the sodium salt of fatty acid used in soap is not synthetic detergent.
New answer posted
3 months agoContributor-Level 9
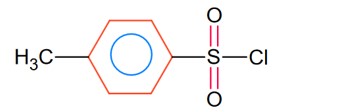
p- toluenesulphonyl chloride
Only gives soluble product with alkali for 1° amine and for 2°- amine it gives insoluble product.
New answer posted
3 months agoContributor-Level 9
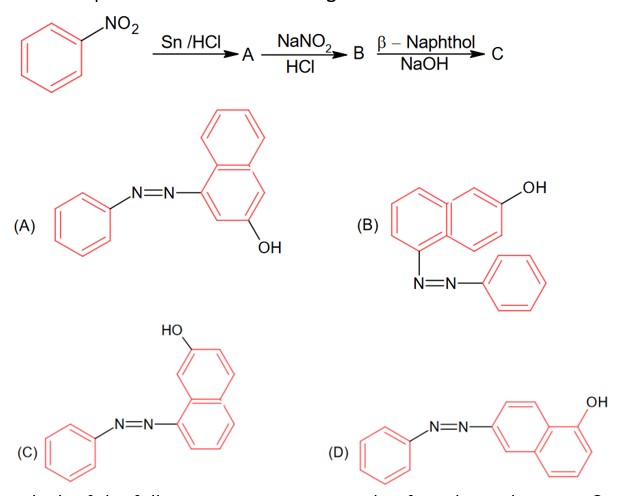
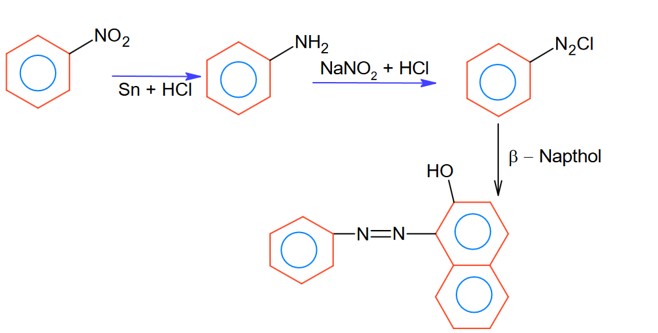
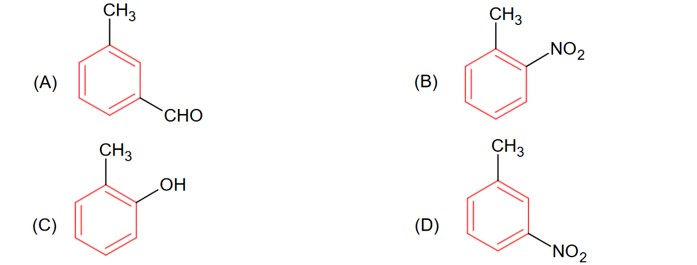
OH group is the more activating group and ortho-para director, hence ortho position attach will provide meta substituted product with respect to – CH3 in option (C).
New answer posted
3 months agoContributor-Level 10
Tautomerism is a dynamic equilibrium between 2 isomers, also known as tautomers, where a compound exists in 2 different structures. The most common form is keto enol tautomerism. It is mainly of two types:
- Keto Form: Contains a C=O (carbonyl) group
- Enol Form: Contains a C=C double bond and an –OH group
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers