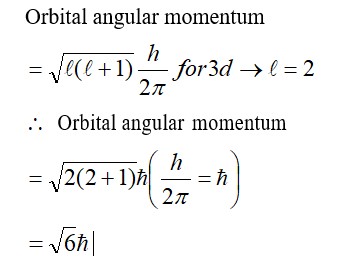
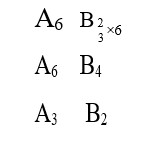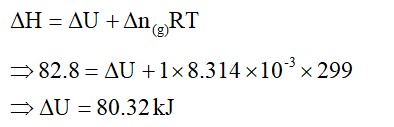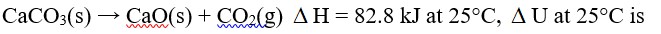Chemistry Thermodynamics
Get insights from 21 questions on Chemistry Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
When any change in matter is not responsible for changing the characteristic of the reaction, the amount of matter we would have intensive properties. These properties, which we know as temperature or density, remain constant even when the matter's size or quantity changes. On the other hand, we would have extensive properties in substances when they change when the amount or quantity in the system changes.
New answer posted
4 months agoContributor-Level 10
Internal energy is the sum of all the energy in the system, as simple as that. But if we look into enthalpy, it is a state function that tells us how much internal energy is there in the system and how much work would be required to do to expand a gas against a constant pressure.
New answer posted
4 months agoContributor-Level 10
For both work and heat, there are positive and negative signs, which mainly depend on transfer of energy. When work is done on the system, it will have a negative sign, but when work is done by the system, we will use a positive sign. This would be similar to how we view heat in chemical thermodynamics. If a system absorbs heat, it will be a positive sign. But if heat releases from a system, it will be a negative sign.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers