When petrol burns, chemical reactions release heat and light. It is a basic principle of thermodynamics that you study first in Chemistry and later in Physics in Class 11. Thermodynamics in chemistry mainly deals with changes in energy. Here, we study the inevitable relationship among heat, energy, and work and how they show up in chemical reactions and how physical states change. To help you with your exams, this article will cover Thermodynamics Class 11 Chemistry notes through relatable examples, the right approaches, and important formulas. So brushing up right before exams will not be a hassle.
What You’ll Learn with Class 11 Thermodynamics Chemistry Notes
- Definitions and differences between the system and the surroundings. From types of systems, states of the systems, to state functions, in relation to the laws of thermodynamics.
- Applications of thermodynamics in chemistry, with work on reversible and irreversible processes in thermodynamics.
- Important measurable concepts in chemical thermodynamics covering calorimetry, meaning and types of enthalpy, along with a deeper understanding of entropy, spontaneity, and Gibbs energy.
- Chemical Thermodynamics: Terms and Concepts to Learn in Class 11
- Applications of Thermodynamics in Class 11 Chemistry
- Calorimetry in Chemistry Class 11
- Change in Enthalpy: Class 11 Chemistry Thermodynamics Notes
- Types of Enthalpies for Reactions
- Spontaneity in Chemical Thermodynamics
- Entropy Definition: Class 11 Chemistry Notes
- Gibbs Energy: Chemistry Notes on Thermodynamics Class 11
Chemical Thermodynamics: Terms and Concepts to Learn in Class 11
If considering how to study Class 11 thermodynamics chemistry, you should begin with the universe where reactions occur, how matter transfers energy in different conditions, and so on.
To put things in perspective as we move ahead, let’s understand one difference in approach when we deal with thermodynamics in chemistry and physics.
In physics, thermodynamics focuses on the system that does work, as in what it gains. It is the system that absorbs heat. So we can have this notion that in physics, it is the work done BY the system.
In chemical thermodynamics, we observe the system from the outside, as in, what we are putting in the system. So, here, the work is done on or put into the system. Also, heat is added to the system. In chemistry, we can say that it is the work done TO the system.
The NCERT textbook introduces one important concept in Section 5.1.1.
“The universe = The system + The surroundings”
System in Thermodynamics
A thermodynamic system is a part of the physical space or matter with a real or hypothetical boundary. We use the laws of thermodynamics to study or analyse it separately from the surroundings.
Types of Systems in Thermodynamics
The types of physical systems in thermodynamics are based on whether they can interact with the surroundings.
- Open System
An open system allows the flow of mass and heat or work (energy) with its surroundings. A basic example is a boiling water pot out in the open, where heat flows out. Another example of an open system is a coffee cup without a lid, which is kept at room temperature.
- Closed System
A closed system does not allow mass transfer with the surroundings, but lets out heat to the surroundings. A pressure cooker with a closed lid is a popular example of a closed system. The mass or contents inside the pressure cooker do not go out, but the heat evaporates.
- Isolated System
An isolated system is one where we do not see any exchange of mass and heat with its surroundings. A thermos flask where hot water remains hot for a long time is one example of an isolated system. Another broader example of an isolated system is our vast and ever-expanding universe where we live in. There is no outside system where it shares or exchanges matter or energy.
State of the System in Thermodynamics in Chemistry
Given what a system is at a given point in time, we can mention that as a thermodynamic state. In order to analyse or measure a state, we need to know the state variables. They are also termed as state functions or macroscopic properties. Pressure, temperature, and volume are the common ones. With these, we can track and/or predict the behaviour of the system as a whole when it changes from one state to another.
Your NCERT book in section 5.1.3 mentions that knowing this helps “describe the system by specifying it before and after the change.”
Difference between State Functions and Path Functions
As we move to bigger concepts in chemical thermodynamics Class 11, we will need to understand a few differences between state functions and path functions.
State functions only define the initial and final states of the system. As in, what it was before and after the change in energy. They are not concerned with what processes went on in the change of state. Technically, we can say that state functions are path independent.
Examples and concepts that you will come across later in the Class 11 Chemistry chapter Thermodynamics notes about state functions are internal energy, enthalpy, Gibbs Energy, temperature, pressure, and volume.
Those which are dependent on the process of converting one state to another are called path functions. Examples here include work and heat.
Internal Energy as a State Function
A thermodynamic system’s internal energy is all the energy inside a system where the molecular movement remains random. In chemistry, we denote the internal energy by U. Some other text sources, besides your NCERT book, may denote it as E.
As said previously, internal energy is an example of a state function. According to NCERT, it is subject to change under three conditions.
“When
- heat passes into or out of the system,
- work is done on or by the system,
- matter enters or leaves the system.”
One of the essential aspects that NCERT stresses is to show you how Internal Energy is a state function.
It brings out some experimental insights based on the First Law of Thermodynamics in Physics, which states that the “energy of an isolated system is constant”. That means, energy is not created, nor can it be destroyed.
∆U= W + q
∆U is the change of internal energy
q is the heat the system receives
W is the work done to the system
This formula for internal energy in chemical thermodynamics is important to understand with respect to the process in an isolated system. Here, the process and the system are both adiabatic in nature.
You must note that this fifth chapter on Thermodynamics in Chemistry Class 11 introduces the adiabatic process that occurs in an isolated system, where there is no transfer of matter or energy to the surroundings. Whatever happens remains in the system. Mathematically, that means, if no heat is transferred, ie, q = 0, the result for the internal energy is only equal to the work done. That also tells us that internal energy is path independent, which means it's a state function.
Also, since it’s work and heat transferred to the system, the IUPAC (International Union of Pure and Applied Chemistry) convention tells us we have to use the positive sign.
Applications of Thermodynamics in Class 11 Chemistry
Here, we recommend you head over to our page on thermodynamics applications. We will provide a snapshot of the basics below.
Pressure-Volume Work
The basic formula for pV work is pressure multiplied by change in volume.
We denote that
W = -pₑₓΔV
We have the negative sign in this pV work only when it is compression. ΔV remains negative, but the work done on the system is positive.
There are two scenarios to understand here, with respect to the important thermodynamics processes - for reversible and irreversible.
For an irreversible process, the work would be
W = -ΣpₑₓΔV
For a reversible process, the work would be
W = -∫pₑₓdV
How to Calculate Work for an Ideal Gas
Your NCERT textbook covers an isothermal reversible process as
wᵣₑᵥ = -nRT ln(Vf/Vi)
In the case of free expansion of gas, there is no work, as there is no external pressure.
Understanding Compression vs Expansion in Chemical Thermodynamics
The calculation of pressure changes with compression and expansion changes. We can use two different formulas for that.
- Compression: pₑₓ = pᵢₙ + dp
- Expansion: pₑₓ = pᵢₙ - dp
Enthalpy (H)
Enthalpy is a state function and is denoted by H. It is the calculation or sum of the internal energy and pressure-volume work.
H = U + pV
At constant pressure: qₚ = ΔH
For an ideal gas, the total change in enthalpy would be
ΔH = ΔU + ΔnRT
Sign Conventions for Enthalpy to Remember
- ΔH is negative when it is an exothermic process. This happens when heat is released.
- ΔH is positive when the process is endothermic, ie, when heat is absorbed.
How are Thermodynamic Properties Classified?
There are two basic thermodynamic properties to learn in Chemistry Class 11.
- Extensive properties: These depend on the amount of matter. These are mass, volume, and enthalpy.
- Intensive properties: These are thermodynamic properties that are independent of amount. Commonly, we refer to temperature, density, and pressure.
Heat Capacity as an Extensive Property
Heat capacity, often denoted as C, is an extensive property. Its value is for a unit mass of a substance in a system. It is important to understand how heat capacity affects the temperature. We will learn that it is the quantity of heat (q) that is transferred to a system. You can refer to the Class 11 NCERT Chemistry book, on page 144, which mentions that we use heat capacity to learn “how to measure heat transferred to a system.”
To calculate it, we get results in a temperature change, ΔT. NCERT also mentions that the “increase of temperature is proportional to the heat transferred” on page 144.
So we use the coefficient C (also the heat capacity), and we mathematically show this relationship or the heat capacity formula as
q = CΔT
What Happens to Heat Capacity for an Ideal Gas?
Here, we need to know the relationship among heat, heat capacity, and state functions when we are considering an ideal gas.
With Constant Volume (Cv)
If there is constant volume, what happens to heat and heat capacity?
The mathematical relationship is,
qV = CVΔT = ΔU
Here is the explanation for it.
When there is no change in volume, there is no expansion work. That is, work or w = 0.
As we learned earlier, energy is constant in an isolated system, as per the first law of thermodynamics. If we use the same 1st Law formula
U = q - w = q - 0 = q
So, it is safe to assume or say that all heat goes into changing internal energy.
CV or Cv is defined as the heat capacity when volume is constant.
With Constant Pressure (Cp)
What if there’s constant pressure, but volume changes?
There would naturally be expansion and work done. Unlike before, when it was constant pressure, there is no work done there, but with volume changing, there will be work.
Mathematically, we can say that work would be
W = pΔV
Using the First Law of Thermodynamics again, we get
ΔU = q - pΔV
Just a simple rearrangement would help us get
q = ΔU + pΔV
If you recall from above, this is the equation for enthalpy at constant pressure.
q = ΔU + pΔV = ΔH
Points to remember quickly:
- Enthalpy (H) equals the heat that is transferrable when pressure is constant.
- But internal energy (U) is equal to the heat transferred at constant volume.
Relationship Between Constant Volume and Constant Pressure for an Ideal Gas
For an ideal gas, we have
Cp - Cv = R
With constant pressure, we know that the heat energy goes into both increasing internal energy as well as doing expansion work against external pressure.
At constant volume, we see that heat energy goes into increasing internal energy, and no expansion work is done.
The difference equals R because that's exactly the amount of extra energy needed per mole per degree to do the expansion work (pΔV) when heating at constant pressure versus constant volume. And these are measured in labs using calorimeters. Follow the next section below.
Calorimetry in Chemistry Class 11
Once we know about heat capacity along with exothermic and endothermic processes in thermodynamics, it is important to learn about calorimetry. It helps us learn how changes in heat help map the two important state functions, enthalpy and internal energy, mathematically.
The process of measuring heat that is either released or absorbed during a chemical reaction or physical change is calorimetry. To calculate this thermodynamic heat exchange, chemists use a calorimeter along with the heat capacity formula.
Constant Pressure Calorimetry vs Constant Volume Calorimetry
Now, there are two types of measurements with calorimetry, which are either used with a closed or an open system. For an open system, the measurement is for constant pressure calorimetry and for a closed system, the measurement is for constant volume calorimetry.
These two help us understand how chemical reactions transfer energy.
Additionally, they let us know which type of measurements are useful for liquid reactions versus gaseous reactions.
-
Constant Pressure Calorimetry
Constant pressure calorimetry is measured with a coffee cup calorimeter, which is like an open system. This device can help measure heat changes that happen at atmospheric pressure, as its system can exchange energy or heat with the surroundings.
-
Constant Volume Calorimetry
For constant volume calorimetry, labs use a bomb calorimeter, which is a closed system. It measures the exchange of heat at a volume that’s fixed.
Change in Enthalpy: Class 11 Chemistry Thermodynamics Notes
This is one of the most essential parts of Thermodynamics Class 11 notes.
From above, we can safely say that we are sure that enthalpy is a state function, and it is the sum of internal energy and pressure-volume work.
H = U + pV
Now, we move on to learning the role of enthalpy in actual chemical transformations. That is, the conversion from reactants to products.
Reactants are those substances before chemical reactions happen. Products are, well, the result.
A basic example would be considering reactants like hydrogen and oxygen that form water as product. The equation looks like this below.
Reactants → Products
Reaction Enthalpy Meaning
In a chemical reaction, there is an enthalpy change when reactants turn into products.
We can say that the enthalpy of reaction is the heat amount that’s either released or absorbed during a chemical reaction. It also depends on the moles that are entirely used up during the chemical reaction to create the product.
Just remember that enthalpy is H, while enthalpy change as ΔH when it comes to a chemical reaction. For reaction enthalpy change, we will denote it as ΔrH.
Mathematically, we can say that the reaction enthalpy is the difference of the sum of molar enthalpies of products and the reactants.
It looks like the equation below.
∆rH = Σ(nproducts × Hproducts) - Σ(nreactants × Hreactants)
- ∆rH is the enthalpy change of reaction
- Σ is the symbol of sigma to denote the sum of all terms inside the brackets
- n is the number of moles, which are also stoichiometric coefficients (in NCERT, n is given as ai and bi)
- H is the molar enthalpy of each substance
Note that molar enthalpy is the heat energy amount that is released or absorbed for every mole of the substance that reacts.
Also, remember that stoichiometric coefficients appear as whole numbers or fractions ahead of a formula in a balanced chemical equation. For instance, the stoichiometric coefficient for 3H₂ would be 3.
Example of Reaction Enthalpy
For 2H₂ + O₂ → 2H₂O
∆rH = [2×H(H₂O)] - [2×H(H₂) + 1×H(O₂)]
Remember, H is the enthalpy in this equation, and should not be confused with Hydrogen.
Important Concepts for Enthalpy Changes
The Thermodynamics chapter for Class 11 Chemistry NCERT highlights a couple of definitions, which are normally asked during exams.
Standard Rate of Enthalpy
“The standard enthalpy of reaction is the enthalpy change for a reaction when all the participating substances are in their standard states. The standard state of a substance at a specified temperature is its pure form at 1 bar.”
This rate has the superscript symbol, ΔH ^ ⦵.
Enthalpy Changes during Phase Transformations
There are three definitions to remember.
Standard or Molar Enthalpy of Fusion
“The enthalpy change that accompanies melting of one mole of a solid substance in
standard state is called standard enthalpy of fusion or molar enthalpy of fusion.”
NCERT example of standard enthalpy of fusion.
“H2O(s) → H2O(l)
∆fusH ^⦵ = 6.00 kJ moI ^–1”
Standard or Molar Enthalpy of Vapourisation
“Amount of heat required to vaporize one mole of a liquid at constant temperature and under standard pressure (1bar) is called its standard enthalpy of vaporization or molar enthalpy of vaporization.”
NCERT example of standard enthalpy of vapourisation.
“H2O(l) → H2O(g); ∆vapH ^ ⦵ = + 40.79 kJ moI^–1
∆vapH^⦵ is the standard enthalpy of vaporisation”
Standard or Molar Enthalpy of Sublimation
“Standard enthalpy of sublimation is the change in enthalpy when one mole of a solid substance sublimes at a constant temperature and under standard pressure (1bar).”
NCERT example:
“Solid CO2 or ‘dry ice’ sublimes at 195K with ∆subH^⦵=25.2 kJ mol^–1.”
Standard Enthalpy of Formation
“The standard enthalpy change for the formation of one mole of a compound from its elements in their most stable states of aggregation (also known as reference states) is called Standard Molar Enthalpy of Formation. Its symbol is ∆f H^⦵.”
NCERT shows the following enthalpies of formation.
“H2(g) + ½O2 (g) → H2O(1);
∆f H ^ ⦵ = –285.8 kJ mol^–1
C (graphite, s) + 2H2(g) → Ch4 (g);
∆f H ^ ⦵= –74.81 kJ mol ^ –1
2C (graphite, s)+3H2 (g)+ ½O2(g) → C2H5OH(1);
∆f H ^ ⦵ = – 277.7kJ mol^–1”
Thermochemical Reactions Definition
The basic definition of a thermochemical reaction is that it’s a balanced chemical reaction that we write together with the reaction enthalpy change (ΔrH).
The SI unit for it will be kJ mol^–1. It is also referred to as the per mole of reaction.
Hess’s Law of Constant Heat Summation
NCERT mentions,
“If a reaction takes place in several steps then its standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions into which the overall reaction may be divided at the same temperature.”
The Hess’s Law has two important conditions that you might remember from above.
- Enthalpy is a state function and an extensive property, as its value depends on internal energy, pressure, and volume, which are state functions themselves.
- As a state function, enthalpy is not dependent on the path. The change in enthalpy (∆H) is not dependent on the path taken from being a reactant to becoming a product.
Types of Enthalpies for Reactions
For chemical reactions of different types, there are different kinds of enthalpies.
Standard Enthalpy of Combustion
Standard enthalpy of combustion is the heat that is released when only one mole of substance burns with oxygen. This reaction must occur at standard conditions, 298 K, and a pressure of 1 bar.
For the standard enthalpy of combustion, we denote it by ∆cH^⦵.
Enthalpy of Atomisation
Enthalpy of atomisation is the change in energy when one mole of a substance in a standard state converts into the state of gas.
∆aH^⦵ is how we denote this.
Bond Enthalpy
Bond enthalpy is also called bond energy. It is the energy required to break one mole of a particular bond in a gaseous molecular form.
It’s denoted by ∆bondH^⦵.
We use bond enthalpy to estimate or predict the enthalpy change of a reaction.
NCERT mentions this approximation and emphasises that it’s present for bonds in the gaseous state.
∆rH (net enthalpy change) ≈ Σ (bond enthalpies of reactants) - Σ (bond enthalpies of products)
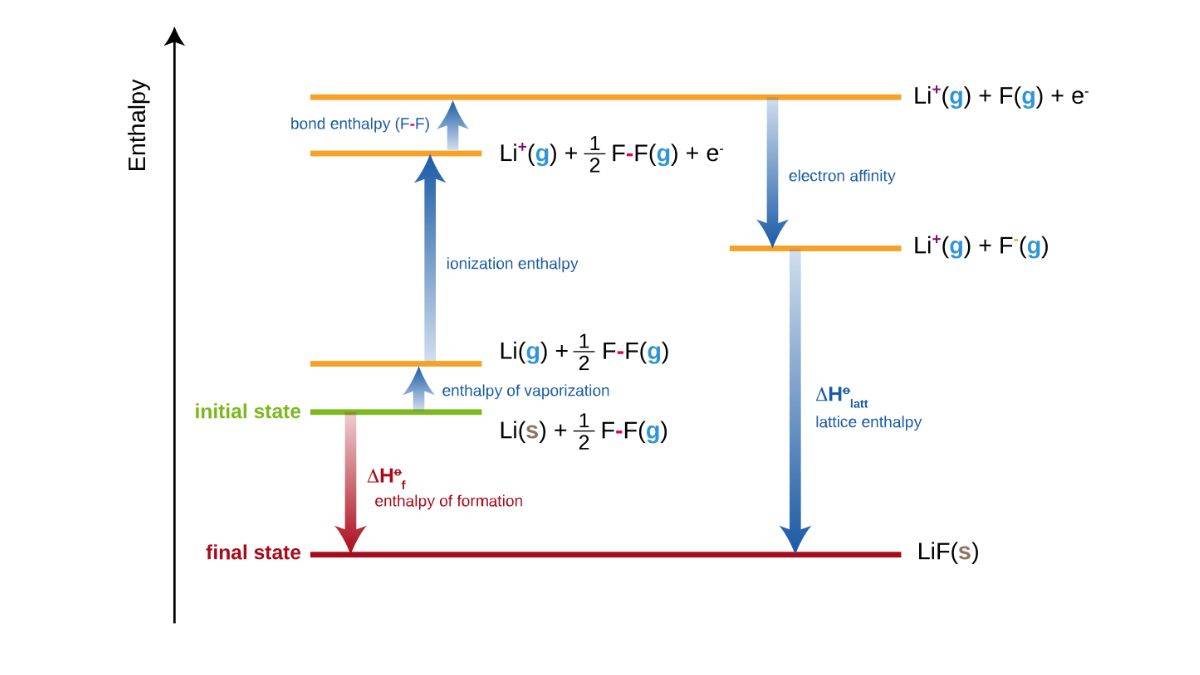
Lattice Enthalpy
It is quite interesting to be so universal that ions have strong electrostatic forces in an ionic solid. Ionic forces are dependent on the charges of ions and the radii of the ions as well. Now, the energy required to separate one mole of a solid ionic compound into gaseous ions is the lattice enthalpy.
How to Calculate Lattice Enthalpy
Lattice enthalpy cannot be calculated directly. We need to use the Born-Haber cycle for that. This cycle is based on the Hess Law, which will let us break down the individual standard enthalpies to get a final sum.
The Born-Haber cycle allows us to construct a cycle of a closed loop of the reactions of sorts. These reactions connect the formation of an ionic solid to how it dissociates into the ions in the gaseous state.
Let’s use an example below, and see the series of steps.
Step 1: Enthalpy of Vapourisation of Lithium
In the beginning state, we have lithium in a solid state. Here, we represent it as Li(s). One mole of this solid is converted into gas, Li(g). Energy is added for it to happen. It’s an endothermic process, as enthalpy here increases.
Step 2: Ionisation Enthalpy of Lithium
After lithium becomes a gas, the atoms absorb energy. By doing so, they lose an electron and form gaseous lithium ions. That forms Li + (g). Even here, enthalpy increases, as it is an endothermic process.
Step 3: Bond Enthalpy of Fluorine
At the same time, we have these diatomic fluorine molecules in gas form, which we know as ½ F₂(g). They absorb energy, break their bonds, to go on to form one mole of gaseous fluorine atoms. That is, F (g).
Step 4: Electron Affinity of Fluorine
The gaseous fluorine atoms gain an electron and form ions. F-(g) is how we would denote this, as this would be showing that F is releasing energy and enthalpy is decreasing. That’s an exothermic process now.
Step 5: Lattice Enthalpy
We can see above that both lithium ions Li + (g) and fluoride ions F - (g) combine to form one mole of solid lithium fluoride. We get LiF(s). A large amount of energy is released. That is the lattice enthalpy we get.
Spontaneity in Chemical Thermodynamics
Spontaneity in thermodynamics tells us that it’s a natural, irreversible process. The product is formed even when it is really quite slow. No external input or continuous energy is required either, for a spontaneous reaction.
By now, we know that a decrease in enthalpy tells us that heat is released as an exothermic process. And ΔH, or the change in enthalpy, becomes negative according to sign conventions. In reality also, we usually observe at room temperature that hot water becomes cooler and becomes not too cold but adapts to reach a state of equilibrium. That is a spontaneous reaction.
But that’s just one part of the puzzle to understand spontaneity in chemistry and thermodynamics.
One of the most important things you must know while learning Class 11 chemical thermodynamics is that not all spontaneous reactions are exothermic. They can be endothermic, and the change in enthalpy would be positive.
A simple chemical reaction of baking soda, which chemistry students know as sodium bicarbonate and vinegar (acetic acid) is spontaneous yet endothermic. This reaction absorbs heat from the surroundings.
Now this is also a contradiction. As enthalpy decreasing is not the only reason for spontaneous reactions. There are two important aspects to keep in mind here. One is entropy, and the other is Gibbs Energy.
Entropy Definition: Class 11 Chemistry Notes
Entropy in chemistry governs randomness or disorder in a system. It is a quantity of thermodynamics that measures the amount of energy that cannot do any more work. And we use the S symbol to denote it.
Since we have been learning most of this topic on the first law of thermodynamics to understand enthalpy and internal energy, we move to the chemical applications of the second and third laws of thermodynamics.
The 2nd Law of Thermodynamics offers a more formalised and universal understanding of entropy. In this law, it’s a physical property or a state variable, including enthalpy, pressure, temperature, and volume, that we can measure. The law states that the entropy state of a universe or an isolated system is bound to increase with time.
If we use maths to define entropy, it would be like
Total Entropy or ∆S = ∆S system + ∆S Surroundings > 0
This is equation 5.19 in your NCERT textbook on page 159.
So when we talk about spontaneity, it is entropy that can explain it. We can also say that for a chemical reaction to be spontaneous, entropy must increase.
What about the change in entropy?
Since we are dealing with chemical applications of the law of thermodynamics, by the third law it is said that the entropy of a pure crystalline substance is zero Kelvin temperature. At this stage or point, there is no movement of atoms in the substance. That’s also why, we need to add some energy like heat.
NCERT on page 159 states that “entropy change is inversely proportional to the temperature. ∆S is related with q and T for a reversible reaction as:
∆S = q_rev/ T
Here, q_rev is either the heat that is released or absorbed in a reversible process. T is the absolute temperature in Kelvin.
Gibbs Energy: Chemistry Notes on Thermodynamics Class 11
Commonly asked questions
Work is not a state function, but why is internal energy one?
This can be a little confusing when we already know the equation from the First Law of Thermodynamics (delta U = q + W) has internal energy as a state function. Work (W) and heat (q) are dependent on the path entirely, but internal energy is only concerned with initial and final states. We can consider the example of work done for an extremely slow or quick process of a gas expanding. The work done for slow work will be different from that of a faster one, but their initial and final states won't have any effect.
Is negative enthalpy the main condition for a reaction to be spontaneous?
We cannot universally say that negative enthalpy is the cause for spontaneity in chemical reactions. Though it is commonly seen in spontaneous reactions that heat releases or follows the exothermic definition, some spontaneous reactions can also absorb heat. They can be endothermic in nature. With that, we get a positive value for enthalpy change. So the better approach is to look into entropy and Gibbs Energy. These two quantities can define or tell us that a chemical process is spontaneous when the total entropy change is positive and when the Gibbs energy change is negative.
Why should we consider enthalpy more important than internal energy when it comes to a chemical reaction?
We consider the enthalpy change (delta H) more important than the internal energy (delta U) for practical reasons. When we go by the definitions alone, internal energy is the heat absorbed or released at a constant volume. This only happens in a sealed container. But in real life experiments held at laboratories, we have open vessels like test tubes, and there, only atmospheric pressure as a state variable remains constant, and not volume per se. This condition of constant pressure becomes more useful to calculate enthalpy in the chemical reaction.
Chemistry Thermodynamics Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics