Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Tl? ³ is less stable than Tl? ¹ (inert pair effect). Going down the group 13, stability of lower oxidation state increases. In case of B, Al, Ga and In, higher O.S. +3 remains more stable than lower O.S. +1 . But, in last stable element, thallium (Tl), lower O.S. +1 become more stable than higher O.S. +3 .
New answer posted
3 months agoContributor-Level 10
The para-magnetism of solution of alkali metals in liquid ammonia and its deep blue color is due to ammoniated electrons. Which absorb energy in visible region of light.
New answer posted
3 months agoContributor-Level 10
Ca plays important role in Neuromuscular function and interneuronal transmission. The daily requirement of Mg and Ca in the human body is estimated to be 200 – 300mg. All enzymes that utilise ATP in phosphate transfer require Mg as the cofactor. The bone in human body is NOT an inert and unchanging substance.
New answer posted
3 months agoContributor-Level 10
A, B and C are correct statements. The H-H bond dissociation enthalpy is the highest for a single bond between two atoms of any element. Hydrogen does not reduce oxides of metals that are more active than iron.
New answer posted
3 months agoContributor-Level 10
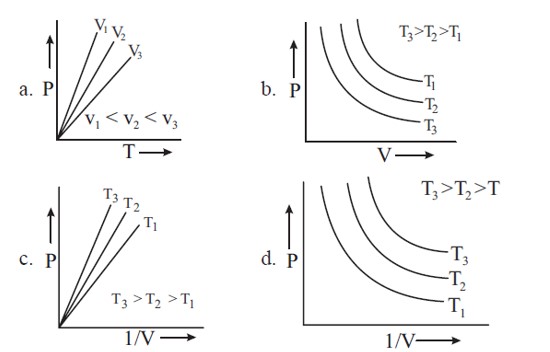
PV = nRT
P = nRT . (1/V)
Plot of P vs (1/V) would be straight line passing through origin having slope = nRT.
At high temperatures, P vs (1/V) would have greater slope.
New answer posted
3 months agoContributor-Level 10
Covalent bonding is NOT an intermolecular force while rest all are considered as intermolecular forces.
New answer posted
3 months agoContributor-Level 10
σ1s < 1s < 2s < 2s < (2px = 2py) < 2pz < (2px = π2py) < *2pz is the correct order of energy of MO for homo nuclear diatomic species N?
New answer posted
3 months agoContributor-Level 10
AlCl? , BeCl? and PCl? does not obey octet rule.
AlCl? and BeCl? both are electron-deficient species having six electrons in valence shell of central atom whereas PCl? has ten electrons in valence shell of phosphorous.
The structures are :
New answer posted
3 months agoContributor-Level 10
Among isoelectronic monoatomic species, size is inversely proportional to atomic number. Hence among isoelectronic species Na? , O²? , N³? , F? (having the nearest noble gas configuration);
Order of size is Na? < F? < O? < N?
N³? has least atomic number hence the largest size
New answer posted
3 months agoContributor-Level 10
nm = 2l + 1
As there are (2l + 1) number of permissible values of magnetic quantum number.
Hence, l = (nm - 1)/2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers