Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Least count = 1 mm / 100 = 0.01 mm
Diameter = main scale reading + circular scale reading
Diameter = 0 + 52 * 0.01 mm
= 0.52 mm = 0.052 cm
New answer posted
3 months agoContributor-Level 10
Kolbe's electrolysis is a process where an aqueous solution of sodium and potassium salt undergoes electrolysis to lead to the formation of a symmetrical alkene. Principle of this technique is that at anode, the carboxylate ion undergoes oxidation and loses carbon dioxide (CO? ). Similarly, at cathode the water decomposes to form hydrogen gas. The alkyl radicals formed in these two cases combine to form alkenes.
New answer posted
3 months agoContributor-Level 10
When nucleoside is linked to phosphoric acid at 5 '-position of sugar moiety, we get nucleotide.
New answer posted
3 months agoContributor-Level 10
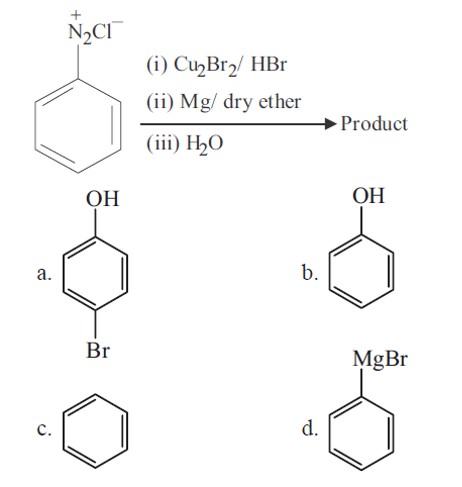
Bromobenzene is formed in first step (Sandmeyer reaction), which further gives phenyl magnesium bromide. Phenyl magnesium bromide further gives benzene with water.
+Mg (Br)OH
New answer posted
3 months agoContributor-Level 10
Helium is used as a diluent for oxygen in modern diving apparatus because of its very low solubility in blood.
New answer posted
3 months agoContributor-Level 10
Sodium ethanoate is CH? COONa and given process is soda-lime decarboxylation.
CH? COONa + NaOH - (CaO)-> CH? + Na? CO?
Methane is obtained having molar mass 16. Two moles would be 32 g.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
In case, nitrogen and sulphur both are present in an organic compound, sodium thiocyanate is formed.
Na + C + N + S → NaSCN
Which further reacts as:
Fe³? + SCN? → [Fe (SCN)]²?
(Blood red color)
New answer posted
3 months agoContributor-Level 10
Coke- reducing agent
Diamond- sp³ carbons
Fullerenes- cage like structure
Graphite- used as lubricant
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers